(1)
where β is the thermal coefficient of volume expansion, T is the temperature rise, and k is the isothermal compressibility. In soft tissue, a temperature rise of 1 mK induces approximately 800 Pa pressure rise, which is above the detection sensitivity of a typical ultrasonic transducer (~77 Pa for a 50 MHz transducer) [12]. Therefore, PAI is a highly sensitive imaging modality. If all absorbed light energy is converted to heat, the initial pressure can be expressed as

(2)
At depths beyond the optical diffusion limit , PAI can provide imaging with high ultrasound resolution, which is determined by the center frequency and bandwidth of the ultrasonic detection system and the bandwidth of the detected PA signal. The higher the central frequency and the broader the bandwidth, the better the spatial resolution. Penetration depths up to 5–6 cm have been reported using a safe laser exposure in the wavelength range of 600–1500 nm [28–30]. PAI scales spatial resolution with penetration depth, as shown in Fig. 1. With a detection central frequency of 50 MHz, acoustic-resolution photoacoustic microscopy has achieved a lateral resolution of 45 μm and an imaging depth of 3 mm [31]. Reducing the detection central frequency to 5 MHz expands the imaging depth to 4 cm and relaxes the resolution to 100–560 μm, depending on the detection geometry [32–35].


Fig. 1
Imaging depth versus spatial resolution in multi-scale PAI . The red circles represent lateral resolution, and the blue triangles denote axial resolution. LA-PACT, linear-array based PA computed tomography [80], PAMac, deep photoacoustic macroscopy [32]; AR-PAM, acoustic resolution photoacoustic microscopy (PAM) [81]; DI-PAM, double-illumination PAM [82]; OR-PAM, optical resolution PAM [14, 15, 83]; 125-MHz-PAM, PAM with a 125 MHz ultrasonic transducer [84]; SM-PAM, submicron PAM [85]; SW-PAM, subwavelength PAM [86]; UV-PAM, ultraviolet PAM [25]; PI-PAM, photo-imprint PAM [87]; SR-PTM/PAM, super-resolution photothermal/photoacoustic microscopy [88]
3 Why Nanoparticles
Without the introduction of exogenous contrast, PAI has been used in a variety of applications, including hemodynamic imaging [3, 36], blood oxygenation quantification [37], blood flow measurement [38], cancer detection [39], and tumor modeling [40, 41]. With appropriately chosen wavelengths, from the ultraviolet (UV) to visible (VIS) spectrum (250–700 nm), PAI can image cell nuclei, cytochromes, and red blood cells with high optical absorption contrast. However, UV and VIS light normally penetrates tissue only for hundreds of microns to millimeters, due to the strong optical attenuation. In the NIR wavelength region (from 700 to 1100 nm), the optical absorption of tissue is at a minimum, which is called “optical window .” The optical scattering in biological tissue decreases with increasing wavelength. Therefore, NIR light suffers the least optical attenuation, which gives PAI the greatest penetration. According to Eq. (2), the low endogenous optical absorption of NIR directly leads to weak PA signals, which affects the imaging sensitivity and depth. Therefore, the employment of exogenous contrast agents can greatly improve the imaging sensitivity, contrast, and specificity. Compared with endogenous molecules, exogenous contrast agents offer several advantages. First, the optical and chemical properties of exogenous contrast agents can be specifically engineered to maximize imaging contrast and detection sensitivity, and to minimize background absorption. Second, exogenous contrast agents can be specifically conjugated with targeting agents (e.g., peptides, antibodies, aptamers) to selectively bind to cell surface receptors. Third, exogenous contrast agents, especially NPs, can be engineered with specific structures for both PAI and localized therapy. For examples, the unique optical properties of these NPs can enable controlled drug delivery by light or ultrasound to the target organs and/or to mediate heat generation for thermal therapy.
A major concern for exogenous PA contrast agents is their photostability. Organic dyes suffer from permanent oxidative photobleaching and optically induced transient changes in their absorption spectrum. They are not suitable for quantitative PA measurement or applications which require high doses of laser energy or a long exposure time. In contrast, metal NPs, such as copper and nickel chloride, silica-coated plasmonic NPs [42, 43], and absorbing pigments, such as melanin from tyrosinase-expressing cells, have much better photostability.
4 Gold Nanostructures
Gold nanostructures, especially gold nanoshells , gold nanocages , gold nanorods , and hollow gold nanospheres , exhibit a unique and tunable optical property, termed surface plasmon resonance (SPR) . SPR is a collective oscillation of conduction-band electrons within the structures induced by the oscillating dipole of a resonant wavelength of light. For solid spherical particles, the resonance peaks appear at approximately 520 nm for gold, and the peak varies slightly depending on the size of the particle and the embedding medium. The SPR peaks of nanostructures can be tuned from the VIS to the NIR region (650–1100 nm) by controlling the size, shape (e.g., nanorods), and structure (e.g., hollow or core–shell structured NPs). These unique optical properties combined with its excellent biocompatibility makes gold nanostructures well suited for PAI of vasculature [44].
Gold nanoshells (AuNS) consist of a dielectric or semiconducting core (i.e., silica) surrounded by an ultrathin gold shell. These nanostructures possess photothermal properties different from solid gold nanospheres of the same size. They have been shown to be useful for a variety of potential applications in photothermal ablation therapy and molecular optical imaging . Wang et al. [45] first reported brain vasculature PAI imaging using AuNS with 100-nm silica cores, 20-nm gold shells, and with an 800-nm peak absorption . A deeply penetrating pulsed laser at 800 nm was employed to image the vasculature architecture of a rat brain. Compared to the brain PAI image based on the endogenous optical contrast, the image acquired ~20 min after three injections of the AuNS showed the brain vasculature with greater clarity. With the exogenous contrast agent , the optical absorption of the blood was increased and the contrast between the vessels and the background brain tissues was enhanced. By ~6 h after the third administration of AuNS, the optical absorption in the blood vessels decreased significantly. This was attributed to the clearance of the AuNS from the blood. The same group further reported high resolution reflection-mode (backward-mode) photoacoustic microscopy (PAM) that noninvasively imaged progressive extravasation and accumulation of AuNS within a solid tumor in vivo [46]. This study took advantage of the strong NIR absorption of AuNS that extravasated from leaky tumor vasculatures via the “enhanced permeability and retention” effect. With PAM , the three-dimensional AuNS distribution inside the tumor was visualized. Experimental results show that AuNS accumulated heterogeneously in tumors, with AuNS concentrated more in the periphery of the tumor and largely absent from the tumor core. This result is consistent with numerous other observations that drug delivery within tumor cores is ineffective because of poor blood perfusion.
Au nanocages are synthesized from Ag nanocubes by a galvanic replacement reaction [47, 48]. Similar to AuNS, the SPR absorption peak of Au nanocages can be tuned throughout the VIS and into the NIR region. Three sequential injections of Au nanocages in rats showed a gradual enhancement of the optical absorption in the cerebral cortex of brain by up to 81 % [47]. Compared with silica-cored AuNS, Au nanocages seem to have advantages for PAI due to their absorption-dominant extinction, more compact sizes (<50 nm), and larger optical absorption cross sections. Gold nanorods have also been employed as contrast enhancement agents for PAI owning to their strong absorption in the NIR range [49–55].
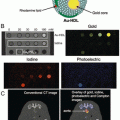
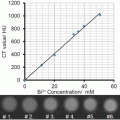
Hollow gold nanospheres (HAuNS ) is a novel gold nanostructure consisting of only a thin Au wall with a hollow interior [56]. This type of gold nanostructures has the unique combination of being small in size (outer diameter, 40–50 nm), having spherical shape, a hollow interior, and a strong and tunable absorption band in the NIR region (Fig. 2). HAuNS are synthesized using cobalt (Co) instead of silver (Ag) NPs as sacrificial templates. The outer diameter of HAuNS is controlled by the diameter of the Co NPs, whereas the interior-cavity size is controlled by the stoichiometric ratio of HAuCl4 and the reducing agents. HAuNS are coated with polyethylene glycol (PEG, MW 5000) to increase their blood circulation half-life. We evaluate the use of these PEGylated HAuNS as a contrast agent for PAI [57]. Our phantom study (Fig. 2d, e) revealed that the PAI of HAuNS was brighter than that of whole blood, aqueous solutions of CuSO4 and India ink. Even though blood had optical density 2.3-fold greater than that of HAuNS in water suspension, the PA brightness of HAuNS was 2.5-fold greater than that of blood, indicating higher PA efficiency of HAuNS than that of blood.


Fig. 2
(a) TEM image of PEG-HAuNS (Bar, 50 nm). The average outer diameter was 45 nm, and thickness of the shell was 2.5 nm. (b) Absorbance spectrum of PEGHAuNS in water, which peaked at 800 nm. (c) Theoretically calculated absorption and scattering spectra of PEG-HAuNS with water core having dielectric permittivity of 1.77. The inner core diameter was 40 nm and gold shell thickness was 2.5 nm, whose values are consistent with those shown in TEM. (d) Tomographic image of a tissue mimicking phantom made of poly(vinyl-chloride) plastisol with four embedded tubes filled with optically absorbing liquid media. (e) Quantitative analysis of the photoacoustic image brightness through the cross-section of each tube from (d). The color scale was designed in three steps: from black to blue/green to red. All tubes with different levels of brightness reflected in the object diameter. Reprinted with permission from Ref. [57]
PAI of the cerebral cortex of a mouse before contrast injection showed only large vessels (e.g., the middle cerebral artery, distributed along the mouse brain cortical surface) (Fig. 3a, arrow). This is because oxyhemoglobin and deoxyhemoglobin display weak PA signal at 800 nm. However, 5 min after the injection of PEGylated HAuNS , the PAI image revealed the brain vasculature with much greater clarity, especially for small blood vessels (Fig. 3b, c). This enhanced clarity was attributed to the strong PA signal generated with HAuNS. This is better appreciated in differential images (Fig. 3d, e) obtained by subtracting the preinjection image from the postinjection images pixel by pixel. At 2 h post-injection, the PA images remained essentially unchanged, indicating a significant amount of HAuNS circulating in the blood (Fig. 3c, e). At higher magnification, PAI revealed not only the structure of large vessels (Fig. 4a, yellow-framed picture) but also small blood vessels (Fig. 4a, green-framed pictures) 2 h after HAuNS injection. Indeed, brain blood vessels in the superficial cortex as small as ~100 μm in diameter could be clearly seen (Fig. 4a, arrows). HAuNS were confirmed to be located on the luminal side of the blood vessels (Fig. 4b, c). There was no particle extravasation into the brain parenchyma. This was attributed to the blood–brain barrier, which has been shown to impede the penetration of such particles. Thus, the long-circulating PEG-HAuNS enhanced the contrast between the blood vessels and the brain parenchyma [57].



Fig. 3
Noninvasive PAI imaging of a mouse brain in vivo employing PEG-HAuNS and NIR light at a wavelength of 800 nm. Photoacoustic image acquired (a) before, (b) 5 min after, and (c) 2 h after the intravenous injection of PEG-HAuNS . (d, e) Differential images that were obtained by subtracting the preinjection image from the post-injection images (Image d = Image b–Image a; Image e = Image c–Image a). Arrow, middle cerebral artery. Bar = 2 mm. (f) Open-skull photograph of the mouse brain cortex obtained after the data acquisition for PAI. Bar = 2 mm. Reprinted with permission from Ref. [57]

Fig. 4
(a) Enhanced PA signals revealed clear and detailed structure of large (yellow-framed picture) and small (green-framed picture) blood vessels in the mouse brain at higher magnification 2 h after intravenous injection of PEG-HAuNS . Arrows represent the small blood vessels with a diameter of about 100 μm, which can be seen in the contrast-enhanced images. (b–d) Distribution of PEG-HAuNS in brain vessels 2 h after injection (Bar = 10 μm). Brain vessels were stained with anti-CD31 antibody (red fluorescence), while the scattering signals of gold particles were detected under a dark field (pseudo-green). Z-stack images showed the particles located on the luminal side of brain blood vessels (b, c). In brain capillaries, three-dimensional reconstruction images show that the particles colocalized or stayed adjacent to the brain capillary endothelial cells (d). Reprinted with permission from Ref. [57]
Integrin αvβ3 is known to be overexpressed in the angiogenic blood vessels of solid tumors. Lu et al. [58] showed that intravenous injection of cyclic peptide c(KRGDf)-coated HAuNS targeting αvβ3 permitted PAI of orthotopically inoculated U87 glioma in nude mice (Fig. 5). Quantitative analysis confirmed that the mean PA signal ratio between the tumor and the contralateral normal brain at 24 h after c(KRGDf)-HAuNS injection was approximately twice as high as that obtained from precontrast images (without contrast agent injection). In comparison, no change in PA signal ratio between the tumor and the normal brain was observed before and after intravenous injection of the nonspecific PEG-HAuNS (Fig. 5). These data indicate that selective binding of c(KRGDf)-HAuNS to both tumor cells and tumor endothelial cells enhanced PAI of U87 glioma. c(KRGDf)-HAuNS also mediated a selective photothermal ablation of U87 tumor, leading to a significant increase in overall survival of tumor-bearing mice compared to mice treated with laser alone or c(KRGDf)-HAuNS alone [58]. These results suggest that PAI may be used to guide photothermal ablation therapy mediated by the same nanoparticle targeted to tumor vasculature and tumor cells.


Fig. 5
(a) Scheme for c(KRGDf)-PEG-HAuNS bioconjugation. (b) c(KRGDf)-PEG-HAuNS characteristics on transmission electron microscopy (bar, 20 nm) and UV–Vis spectrum (measured in water). (c) PAI images of U87 human glioma in mouse brains before (0 h) and 24 hours after i.v. injection of NPs (bar, 5 mm). Photographs of corresponding mouse brains were used to confirm tumor location. Arrows, locations of tumors; L left. (d) PA signal intensity ratio of tumor-to-contralateral brain in mice before (0 h) and 24 h after injection of HAuNS. Data arepresented as mean = SD. c(KRGDf)-PEG-HAuNS group, n = 5; PEG-HAuNS group, n = 4. *, significant difference between precontrast and 24-h postcontrast groups (p < 0.05). Reprinted with permission from Ref. [58]
By integrating multiple functions into a NP’s design, it is possible to use the same nanostructure for multimodality imaging applications. Along this line of research, Zhou et al. [59] reported dual PAI and magnetic resonance imaging (MRI) after intravenous administration of superparamagnetic iron oxide (SPIO)-containing gold nanoshells (SPIO@AuNS). Here, PAI was used to delineate tumor vasculature, while T2-weighted MRI was used to monitor therapeutic effects after photothermal therapy mediated by SPIO@AuNS. Gadolinium (Gd)-doped, gold-speckled silica NPs were synthesized as multimodal nanoparticulate contrast agents for noninvasive imaging using both PAI and T1-weighted MRI [60].
5 Copper Sulfide Nanoparticles and Carbon Nanotubes
NIR light at 1064 nm lies on the second window in the NIR region that encounters less tissue attenuation by the absorption of hemoglobin and water. Among the pulsed lasers emitting NIR light, the Q-switched Nd:YAG laser, which emits laser light at 1064 nm, can provide high pulsed energy with a nanosecond pulsed widths. In fact, its second harmonic radiation at 532 nm is usually employed to pump other lasing media, such as Ti:Sapphire, to obtain tunable laser output in the NIR region. The laser energy conversion efficiency is characteristically around 10 % (~50 % of second harmonic generation and ~20 % of other lasing media). This means that with a typical laser output of ~1 J/pulse at 1064 nm from a commercial Q-switched Nd:YAG laser, only ~100 mJ/pulse maximum output can be obtained at 800 nm, which is within the first biological tissue window. With 10 times greater available laser pulse energy, a 1064-nm laser would have a much higher fluence rate than that of an 800-nm laser pulse (which is generated with a 532-nm pump and an additional lasing medium) at a depth ≤50 mm. Higher laser energy should translate to a stronger PA signal, higher SNR, and greater field-of-view. However, at 1064 nm, blood vasculature or tumor cells have little specific optical absorption that clearly distinguishes them from other normal organ structures.
Towards the goal of enhancing the sensitivity and specificity of PAI at the 1064 nm wavelength, we recently reported the use of copper sulfide NPs (CuS NPs) as a new class of contrast agent for deep-tissue PAI. CuS NPs display optical absorption tunable to 1064 nm and are much smaller (diameter <15 nm) than plasmonic Au nanostructures, which should be preferable for imaging extravascular tumor cells [61]. CuS NPs are readily synthesized in aqueous solution by reacting CuCl2 and Na2S in the presence of various stabilizers [62, 63]. Preliminary PAI studies were conducted with PEG-coated CuS NPs dispersed in 10 % polyacrylamide gel embedded in fresh chicken breast. The CuS NP targets in this phantom could be clearly visualized at ∼5 cm depth from the laser irradiation surface. Figure 6 shows in vivo PAI images of a mouse brain acquired with green light at 532 nm without exogenous contrast (Fig. 6a) and with NIR light at 1064 nm using PEGylated CuS NPs as a contrast agent (Fig. 6b, c). Hemoglobin is the major chromophore in biological tissues and has strong absorption of green light at 532 nm. As has been shown before with PEG-HAuNS (Fig. 5), the superficial vascular structures of the mouse brain, such as the veins and arteries in the cerebral and temporal lobes, could be clearly visible with green light (Fig. 6a). However, green light cannot penetrate deeply because of strong tissue absorption and scattering at short wavelengths. On PAI images of the mouse brain acquired at 1064 nm, only the sagittal and transverse sinuses were visualized; blood vessels were not discernible because of the lack of contrast between blood vessels and the brain parenchyma (Fig. 6b, c). A nodule on the left cerebral cortex that was injected intracranially with 15 μL of an aqueous solution of CuS NPs (2 OD) 24 h before PAI acquisition was clearly seen, which dissolved 7 days after CuS NP injection because CuS NPs were cleared from the injection site (Fig. 6b, c). These studies confirm that CuS NPs were effective PAI contrast agent at 1064 nm and may be used for molecular PAI imaging of tumor and angiogenic blood vessels when these NPs are decorated with appropriate ligands.


Fig. 6
Representative in vivo PAI images of a mouse brain. Images were acquired using laser light (a) at a wavelength of 532 nm without exogenous contrast, (b) at 1064 nm 24 h after intracranial injection of 15 μL of CuS NP solution, and (c) at 1064 nm 7 days after intracranial injection of 15 μL of CuS NP solution. (d) Photograph of the head of the mouse. Laser light was delivered from the top. Reprinted with permission from Ref. [61]
The blood vessel network of a tumor regulates the supply of nutrients and oxygen to the cancer cells, affects their survival and growth, and influences the response of the tumor to therapy. Noninvasive in vivo studies of the tumor blood vasculature are therefore of interest in fundamental cancer research and the development of new drugs and other therapies. To test whether PEG-CuS NPs can be used as an exogenous contrast for PAI of tumor vasculature, we used the orthotopic 4 T1 mammary tumor model. Without contrast agent, several large vessels could be seen, probably due to light absorption by hemoglobin and water molecules at 1064 nm (Fig. 7a) [64]. However, smaller blood vessels are not discernible. At 5 min and 2 h after intravenous injection of PEG-CuS NPs (2 OD), blood vessel structures, including smaller vessels, were more clearly visualized (Fig. 7b, c), indicating increased vasculature contrast after PEG-CuS NP injection. PAI images acquired at 1064 nm with PEG-CuS NPs suggest that this contrast agent is a promising platform for PAI of tumor blood vessels. An interesting aspect of the studies by Zhou et al. [64] was the incorporation of 64Cu, a positron emitter to the same CuS NP matrix without the use of any radiometal chelator, which permitted simultaneous micro-positron emission tomography (μPET) imaging and quantification of tumor uptake of PEG-CuS NPs. Such a dual-modality imaging approach enabled by a single contrast agent is expected to provide much needed complimentary information that cannot be readily acquired with a single imaging modality.


Fig. 7
Representative in vivo PAI images of a 4T1 mammary tumor grown in the mammary fatpad and corresponding PA signals (n = 3). Images were acquired using ns-pulsed laser light at a wavelength of 1064 nm (a) before CuS NP injection, (b) 5 min after intravenous injection of 200 μL of CuS NP solution (100 μg mL − 1, 2 OD), and (c) 2 h after intravenous injection of 200 μL of CuS NP solution. (d–f) PA signal traces correspond to the white lines in the respective PA images. Reprinted with permission from Ref. [64]
Several other attempts have been made to increase signal intensity for PAI contrast agents. For example, conjugating small optical dye molecules to single-walled carbon nanotubes (SWNT-dye) led to sub-nanomolar sensitivity for PAI [65]. The dyes used for conjugation includes indocyanine green (ICG), methylene blue (MB), QSY21 (QSY), and cyanine (Cy5.5). Studies have shown that dye-containing SWNT had much higher optical absorption in the far red and NIR region than SWNT without dyes. Tumors in mice injected with RGD peptide-coated, dye-conjugated SWNT targeted integrin αvβ3 in angiogenic blood vessels, leading to a threefold increase in PA signal intensity compared to tumors in mice injected with the untargeted SWNT-dye [65]. These and other studies demonstrate that with appropriate nanoparticle design, it is possible to improve the sensitivity of PAI to picomolar concentration [51, 57, 66].
6 PA Imaging of Sentinel Lymph Nodes and Atherosclerotic Plaques
In addition to imaging vasculature and tumor blood vessels, various gold nanostructures are also studied as possible PAI contrast agents for mapping of sentinel lymph nodes (SLNs) [48, 55], ex vivo imaging of joint tissue [67], monitoring of drug release [68], and characterization of macrophages in atherosclerotic plaques [69, 70]. PAI of SLNs is worth noting in particular. The SLN, the first lymph node receiving drainage from the tumor is believed to be most likely to be positive for metastases. SLN biopsy has been used in the clinic as the preferred method for tumor staging. Before a biopsy can be taken for staging purposes, SLN must be identified. Current methods for SLN mapping typically use a blue dye (e.g., isosulfan blue or methylene blue) or radioactive colloids (e.g., technetium-99 or 99mTc). However, these approaches require either invasive techniques to visualize the blue stain or specialized facilities to deal with potentially hazardous radioactive components. Therefore, techniques such as PAI that can map SLNs without surgery or radioactivity are highly desirable. Using Au nanocages as the PAI contrast agent, Song et al. [48] have shown that SLNs could be readily imaged with PAI in the context of the surrounding vasculature and within ~3 mm below the skin surface of a rat (Fig. 8). By placing chicken breast tissue on top of the rat skin, a further study showed that the SLN could be imaged with good contrast as deep as 33 mm below the skin surface, which is significantly deeper than the ~12 mm depth of SLNs in humans.