Fig. 1
3D time-of-flight (TOF) MR cholangiogram in a rat following intravenous injection of gadolinium-functionalized perfluorooctylbromide nanoparticles (NP). (a) Baseline image showing no evidence of vasculature or common bile duct. (b) 1 min post-injection, the blood pool as seen in the heart, aorta (arrow), and peripheral vasculature have strong T1-weighted positive contrast from the NP that are still constrained within the vasculature. H heart, L liver. (c) Within 5 min, the NP are rapidly excreted through the common bile duct (arrows), reflecting the rapid shunt of contrast from the liver into the small intestine. (d). After 10 min the small intestine contains most of the contrast, that is passed on to the large intestines at 30 min (e) and 60 min (f). Reproduced with permission from Ref. [22]
3 Spectral CT Contrast Agents
Many CT contrast agents have been created using metals with K-edge values within the X-ray energy bandwidth and the vast majority have crystalline cores that exceed 10 nm. While ultimately these types of agents may prove safe and effective, the scope of the present review generally considered small crystalline nanoparticles (<10 nm) and large degradable nanoparticles encapsulating small molecule organometallic complexes or small solid nanoparticles (<10 nm).
From a spectral CT perspective, two independent blood pool contrast agents imaged simultaneously may have important medical utility; however, the use of a single blood pool agent will likely have limited differential benefit over current iodine based approaches. For new contrast technologies to gain clinical and economic traction, they must address important unmet functional or biochemical (i.e., molecular) imaging needs. In some situations, this may be achieved through in situ labeling of cells, e.g., macrophages in inflammatory imaging, and in other situations by ligand-directed targeting. Regardless, the development of a molecular imaging agent should begin with a clear understanding of the unmet need, which then defines parameters for pharmaceutical design.
4 Bismuth
4.1 Organobismuth Nanocolloid (NanoK)
Emergency departments in the USA assess more than 8 million patients annually with complaints of chest pain or shortness of breath [28]. While a presumptive diagnosis of life-threatening acute coronary syndrome (ACS) may be apparent from the patient’s initial presentation, the vast majorities of cases are equivocal and require the potential of cardiac involvement to be excluded. Consequently, individuals are retained for close observation and testing. For patient without cardiovascular symptoms, there is inconvenience, family stress, and high healthcare cost. Patients admitted for possible ACS require continuous cardiac telemetry, serial ECGs, and repeated cardiac troponin assays over 12–24 h. If cardiac infarction and unstable angina are excluded, then noninvasive cardiac stress testing is typically performed.
Coronary CT angiography has become increasingly refined and offers the potential to recognize patients with low likelihood of disease but the negative and positive predictive values do not meet the standard set by cardiac catheterization [29]. Moreover, coronary calcium, due to its attenuating and blooming artifacts, further complicates the clarity of the angiogram in the region of potentially critical disease [30]. Spectral CT contrast imaging offers an opportunity to target and detect intraluminal thrombus associated with acutely ruptured plaque while discriminating between attenuation artifacts of calcium deposits, which have low (K-edge energies (4.4 keV) (Fig. 2).


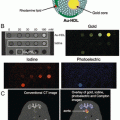
Fig. 2
Example of spectral CT using a coronary vascular example to illustrate the discrimination of calcium and spectral CT contrast agents; (a) coronary artery with a partial occluding thrombus emerging from rupture of the unstable intimal cap. (b) fibrin targeted with bismuth nanocolloid (BiNC) with in classic CT (photoelectric) image and attenuation due to calcium are seen (c) spectral CT (K-edge image) discriminates the fibrin-targeted BiNC from the calcium deposits, (d) integration of the classic CT X-ray image with the spectral CT molecular imaging result acquired simultaneously, reveals the signal from the fibrin-bound bismuth resolved from the atherosclerotic calcium deposits. Reproduced with permission from Ref. [31]
An initial approach to this medical imaging problem was reported by Pan et al. [31] using nanocolloids comprised of high concentrations of an organobismuth compound, bismuth neodecanoate, commixed in sorbitan sesquioleate. The phospholipid-encapsulated “soft” nanocolloid was 20 % w/v aqueous suspension with hydrodynamic diameters between 180 and 250 nm, a negative electrophoretic potential ranging from −20 to −27 mV, and 1.06 g/ml. The particle core was 12–14 wt% bismuth. The biocompatible outer lipid membrane can be functionalized for ligand-direct (peptide and antibody) homing to microthrombus (Fig. 3).


Fig. 3
Synthesis and physicochemical characterization of NanoK: (a) Schematic describing the preparation of bismuth-enriched K-edge nanocolloid (NanoK (Bi)): (b) characterization table for three replicates of NanoK; (c) hydrodynamic particle size distribution from dynamic light scattering (DLS); (d) anhydrous state TEM images (staining: uranyl acetate; scale bar: 100 nm; (e) Atomic force microscopy image (deposited on glass substrate). Reproduced with permission from Ref. [31]
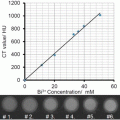
In vitro studies with the bismuth nanocolloid compared cross-sectional CT (conventional) and spectral CT images of phantoms containing serially diluted bismuth particles and a reference calcium acetate suspension in water. This illustrated the potential of K-edge imaging to segment calcium from bismuth nanocolloid despite the X-ray attenuation similarity of the materials at 60 keV, (Ca: 1060HU, Bi: 1050HU). As expected the attenuation of the bismuth formulation varied linearly with the element concentration (R 2 = 0.999).
Additionally, fibrin-targeted bismuth nanocolloid revealed excellent delineation and signal enhancement on spectral CT images of fibrin clot phantoms embedded with calcium. The control clot treated with targeted nonmetallic nanoparticles had negligible contrast, exhibiting only the highly attenuating calcium. The specific targeting of rhodamine-labeled bismuth particles to fibrin on human carotid endarterectomy specimens was corroborated microscopically with immunohistochemistry [31] and exemplified how the K-edge agent targeting was constrained to intravascular rather than intramural fibrin deposits. Dynamic imaging of NanoK with a clinical multidetector CT revealed that blood pool background was no longer detectable after 15–30 min, which would permit optimal molecular imaging of intraluminal thrombus after 1–2 h. The antibody-targeted fibrin bismuth agent concentrated in situ over the acute thrombus in 30 min, remained bound to the clot during 90 min continuous blood circulation, and was detected with a first generation spectral CT scanner as a partial-occlusive thrombus within a 1.41 mm diameter artery, equivalent to a small coronary artery in humans. The bismuth-enhanced clot was clearly differentiated within the vessel and distinct from adjacent attenuation effects of the femur. Superimposition of the simultaneously acquired K-edge image with the traditional CT image spatially oriented and localized the intravascular lesion relative to the rabbit’s skeletal anatomy and demonstrated the complementarity of spectral CT molecular imaging and traditional CT [31] (Fig. 4).


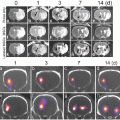
Fig. 4
(a) CT blood pool signal in rabbits following IV injection of NanoK. Inset shows the concentration of bismuth (ICP) in blood versus time post injection. Note that the background signal is at baseline in less than 30 min; (b, c) targeting in situ clot (thrombus) in rabbits (arrow indicates thrombus); (Scale: maximum clot diameter = 1.41 mm; minimum diameter = 1.25 mm). (d) Two weeks clearance profile of bismuth from mice. Reproduced with permission from Ref. [31]
Importantly, whole-body bioelimination of bismuth following intravenous injection was studied in adult male BALB/c mice in a 2-week pilot study. Nearly all of the metal was cleared from the mice within 14 days, and most was bioeliminated by the first week. The residual biodistribution on day 14 into primary particle clearance organs (i.e., liver, spleen, kidney) was assessed using inductively coupled plasma optical emission spectrometry in a second cohort of mice (n = 3), which revealed less than 10 ppb (i.e., the lower detection limit). A third cohort of mice assessed the in vivo impact of bismuth nanocolloid and saline on liver (ALT, AST, albumin) and renal (BUN, CR, Na, K, Cl) function was assessed on days 1, 7, and 14 post-injection. All tests in both groups remained within accepted normal limits for each parameter and no statistical differences between groups were noted at any time point. The overarching conclusions from this early work were: (1) that the nanocolloid offered stable, detectable bismuth contrast for imaging, (2) that the organobismuth complexes could be bioeliminated, and (3) that the nanosystem acute toxicology was favorable [31].
4.2 Bismuth Sulfate: Alginate Microcapsules
The concept and benefit of small molecule bismuth contrast entrapped within larger particles or microcapsules was instituted by Barnett et al. [32]. These investigators addressed the challenge of pancreatic islet cells transplantation by entrapping the cells and within a permeability selective bismuth-alginate capsule. The semipermeable capsule accommodated the influx of glucose and the responsive efflux of insulin, while excluding the penetration of humoral and cell-mediated immune factors. In this context, the bismuth-doped alginate offers radiopaque contrast to both guide implantation as well as support longitudinal monitoring capsule persistence. Upon the eventual metabolism of the capsule, the alginate and bismuth are metabolized or eliminated via urinary excretion. Traditional CT imaging provided very good spatial localization of the implants, however, spectral CT could provide quantitative estimates of bismuth content. Estimates of bismuth content can be converted into more precise assessment of microcapsule numbers and at least qualitative projections of islet numbers the adequacy of their insulin response to glucose challenge. Functional longitudinal monitoring afforded by quantitative spectral CT would clearly facilitate improved clinical management of these pancreatic surrogates.
4.3 Bismuth Sulfide Nanodots
Large-scale synthesis of bismuth sulfide (Bi2S3) nanodots was reported by Lu [33]. In contradistinction to the report from the Weissleder lab [34] using large crystalline bismuth particles, these nanodots had uniform particle sizes approximately 2–3 nm, well below the renal clearance threshold. Although current production methods at that time for hydrophobic Bi2S3 nanoparticles lacked effective surface modification methods, Ai et al. coated the nanodots with either poly (vinylpyrrolidone) (PVP) for stability and biocompatibility. PVP-bismuth nanodots were compared in vivo to Iobitridol™, an X-ray contrast molecule with 45.6 wt% iodine. The intravenously administered nanodots circulated for 1 h and accumulated rapidly in the liver and spleen. Little bismuth was observed clearing via the kidneys into the bladder. Since these tiny particles were quickly sequestered into the mononuclear phagocyte system (MPS) , one might infer that they aggregated in circulation. Subsequent histology showed no gross changes to clearance organ microanatomy, which is consistent with the expected low toxicity of nonionized bismuth. The Iobitridol™ control produced a very brief blood pool contrast then cleared through the kidney into the urine within 3 min. While prolonged blood pool contrast can be beneficial, particularly for techiques like MRI, a typical clinical CT imaging sequence is concluded in seconds, diminishing this advantage.
4.4 Dendrimeric Bismuth Sulfide
An alternative approach to coated minute bismuth nanoparticles was offered by the Shi laboratory, which reported the development of dendrimer-bismuth sulfide [35]. The agent was produced by reacting Bi3+ with generation 4 poly(amidoamine) dendrimers (G4.NGlyOH) followed by exposure to hydrogen sulfide to generate dendrimer coated-Bi2S3 nanoparticles, sized 5.2–5.7 nm. As expected, the X-ray contrast of dendrimer-bismuth sulfide was greater than that of iodine on an equimolar metal basis. The complex had minimal cellular cytotoxicity and good hemocompatibility. When injected subcutaneously in a rabbit thigh, the localized complex was readily appreciated with CT. However, when administered systemically in mice, the enhancement seen within the pulmonary vein and aorta was only slightly greater than an equimolar dosage of Omnipaque™, a commercially available iodine contrast agent. No pharmacokinetic, biodistribution, and bioelimination data of the dendrimer-bismuth sulfide were provided.
5 Gold
Gold nanoparticles and nanoshells have been extensively studied as optical, photoacoustic, and more recently X-ray contrast agents. Indeed the use of gold for optical contrast, particularly photoacoustic contrast agents , precedes later reports of its use for CT contrast nanoparticles, although it is long known to be X-ray attenuating. Many of the gold-based agents used for photoacoustic imaging are relatively large and exceed the renal threshold for clearance, but some are comprised of small gold nanoparticles alone or encased in larger nanoparticles.
5.1 Gold Nanobeacons
One important indication for nanotechnology has been the need for image-guided sentinel lymph node biopsy (SLNB) in patients with breast cancer and melanoma to stage metastases and avoid more invasive biopsy. Invasive SLNB can lead to seroma formation, lymphedema, sensory nerve injury, and limitation in the range of motion. Alternative noninvasive methods to identify sentinel lymph nodes (SLNs) in conjunction with either minimally invasive percutaneous fine-needle biopsy or molecular techniques could offer new prospects for noninvasive axillary staging of breast cancer. Pan et al. demonstrated the use of gold nanobeacons with varying gold nanoparticle content in the context of photoacoustic imaging; these gold nanobeacons were demonstrated to be highly effective for the same application with spectral CT [36–38]. The initial gold nanobeacons were designed as the encapsulation of octanethiol coated metallic gold nanoparticles (2–4 nm) suspended in vegetable and encapsulated with phospholipid with a nominal hydrodynamic diameter of 154 ± 10 nm. The polydispersity and zeta potential were measured to be 0.08 ± 0.03 and −47 ± 7 mV, respectively. Gold content, as determined by inductively coupled emission mass spectrometry, was 1080 μg/g of the 20 % colloid suspension (of GNB160). For SLN mapping, GNB160 was compared with a polymer-encapsulated gold nanobeacons (GNB290) developed through the self-assembly of amphiphilic di-block copolymer in aqueous media to entrap high payloads of gold. The same octanethiol coated AuNPs (2 w/v%) were suspended in polysorbate and microfluidized with a PS-b-PAA dispersion to obtain the GNB290 particles (particle size: 289 ± 24 nm, polydispersity index: 0.15 ± 0.04, gold: 134 μg/g or ~71,493 gold atoms per nanobeacon. While the original GNB was effective for SLN imaging, GNB290 was not due to its weight, inhibiting uptake and migration through the lymphatics following intradermal injection into the forepaw. Consequently, a smaller lipid-encapsulated (~90 nm) gold nanobeacon (GNB90) was produced by suspending octanethiol-functionalized, coated AuNPs (2 w/v% of inner matrix) in polysorbate then microfluidizing the mixture with phospholipid-based surfactant. The GNB90 particle size was 92 ± 12 nm with a polydispersity of 0.35 ± 0.05. The gold content was only 1.56 μg/g or approximately 9 gold metal atoms per GNB90. This particle and smaller payload rapidly transited from the forepaw intradermal injection site through the lymphatics into the SLN within a few minutes. The extent of lymph node uptake of the GNB90 particle far exceeded the original signal appreciated with GNB160 nanocolloid. For SLN imaging, it was evident that smaller particles with less gold/particle yielded more PA signal.
SLN mapping using GNB90 was next evaluated with spectral CT using an analogous experiment. As shown, the magnitude of the spectral CT image was dramatic, reflecting the high mass of gold accumulated rapidly into the node. The “soft” nature of the colloid, the incorporation of very small gold particles, and the very limited gold dose required for SLN imaging all contributed to what could be a translatable safe and effective formulation for this application [39] (Fig. 5).


Fig. 5
In vivo noninvasive spectral CT imaging of SLN. (a) A cartoon illustrating the site of injection and the area of interest. 150 ml of nanobeacons were injected intradermally. (b) regional SLN were clearly contrasted on conventional CT. (c) K-edge contrast of accumulated gold nanoparticles in the SLN was selectively imaged with spectral CT. Reproduced with permission from Ref. [39]
5.2 High-Density Lipoprotein Gold Nanoparticles
The quest for distinguishing vulnerable versus stable atherosclerotic plaque prior to rupture remains a daunting challenge and unmet need in cardiovascular medicine. Since the early work of Benson [40] and Constantinides [41], the acute formation of thrombus following atherosclerotic plaque rupture has been well recognized as the etiology of unstable angina, myocardial infarction, transient ischemic attacks and stroke [42, 43]. Sensitive detection and differentiation of vulnerable versus stable atherosclerotic plaques in vessels with mild severity stenoses remains limited. As the PROSPECT trial showed, the current best invasive imaging technologies, i.e., angiograms and intravascular ultrasound, predict future regions of plaque rupture poorly, even in high-risk patients presenting with myocardial infarction [44]. While still inadequately understood, the risk of plaque rupture is primarily related to the composition of plaque, and considerable noninvasive imaging effort has been directed to characterizing the atherosclerotic inflammatory component.
In this regard, Cormode et al. developed a gold-enriched HDL nanoparticle mimetic as a relatively specific approach to labeling and identifying macrophage-rich plaques in the aortas of apoprotein E knock-out mice (APOe−/e−) using micro-CT [45]. As previously discussed plaque is often laden with calcium deposits, which like gold has marked X-ray attenuation that can be differentiated with spectral CT. Moreover, spectral CT was employed to differentiate the uptake of the gold HDL particles in APOe−/e− mice from the iodine contrast enhancement of the aortic lumen [46, 47]. Although these first-generation photon-counting scanners had very slow image acquisition speeds unable to support live animal studies, this seminal report offered the first in vivo illustration of multi-spectral imaging potential in a pathological animal model. Moreover, in contradistinction to earlier versions of this gold-HDL approach, the nanosystem used in this study incorporated gold particles of 3 nm with the overall diameter of the HDL particle around 7 nm, basically one gold particle per HDL particle. The dose of gold used was around 500 mg/kg, somewhat higher than a typical iodine contrast load (370 mg/kg). Given the small size of the gold particles, they have the potential for renal clearance, but given the dosage of particles required for in situ macrophage labeling , the long-term bioelimination of the metal will need to be better characterized.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree