Fig. 1
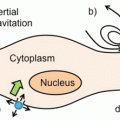
Approaches to labeling stem cells . Panel a shows direct imaging in which an exogenous material is placed inside a cell. The cells are then purified and implanted into an animal. Over time, the amount of contrast agent per cells decreases as the cells divide. In contrast, in indirect imaging (b), a reporter gene is placed inside the cell. These reporter genes either produce a label or affect an injected substrate that increases contrast in the cells of interest. Because the reporter gene is an inherent part of the cells’ biology, this reporter is passed to all progeny with no dilution effects. However, a substrate has to be repeatedly injected for each imaging event. Reproduced with permission from Ref. [12]
Cell-Based Therapy in History
Some of the first reported instances of cell-based therapy were done to counteract the aging process. The French physician Charles-Édouard Brown-Séquard was known to inject pulverized animal testicles into human subjects. Unfortunately, little long-term impact was ever noted. In 1931 Dr. Paul Niehans treated an athymic patient with cells from a bovine thyroid. Although these therapies were also failures because of immune differences between species, his work was visionary, and he described “a method of treating the whole organism on a biological basis, capable of revitalizing the human organism with trillions of cells by bringing to it those embryonic or young cells which it needs.” During the 1950s and 1960s bone marrow transplantation became increasingly sophisticated and matured from grafts between identical twins to grafts between siblings as the knowledge about graft-versus-host disease increased. The first transplant between unrelated persons was in 1973, which led in time to the transplantation of entire organs. The current state of the art is stem cell therapy , which uses cells capable of diverse lineages to repair tissue.
In direct labeling, cells are tagged with small molecules including radioisotopes, fluorophores, and nanoparticles. These can be added during expansion in tissue culture or immediately before injection. Transfection reagents may be used to increase the efficiency of label uptake. The labels can either be on the cell surface or inside the cell. One advantage of intracellular labeling is that there is a reduced chance of the label becoming disassociated from the cell and contributing to artificially high background or erroneous signal.
Direct labeling is attractive because it is simple and straightforward to control the dose of contrast with short processing times [12]. The major limitations are dilution of the concentration of contrast with successive cell division—that is, each daughter cell only has 50 % of the amount of label as the parent cell (Fig. 1). In addition, these labels are usually “always on.” They will report the presence of cells even if the cells are dead. These direct labels can also be taken up by macrophages after cells have died, which can also contribute to inaccurate cell counts.
Common examples include lipophilic fluorophores for optical imaging and (carboxy)dextran-coated super paramagnetic iron oxide (SPIO) nanoparticles such as Feridex® and Resovist® for MRI [13]. While cell loading is traditionally done ex vivo, one interesting report showed that i.v.-injected SPIO nanoparticles can accumulate in the bone marrow and label stem cells in the bone marrow in vivo through the reticuloendothelial system [14]. Fluorescent dyes have value in small animal models, but humans have too much optical scatter for direct optical labels. Radionuclides include fluorodeoxyglucose (18F-FDG) in PET and 111In oxine for single-photon emission computed tomography (SPECT), but one limitation of nuclear imaging methods is that the radioisotope decays over time making it difficult to perform longitudinal scans using radionuclides. More detailed descriptions of direct labels can be found elsewhere [15].
One area of imaging that has been significantly overlooked for cell tracking is ultrasound , which is somewhat surprising because ultrasound offers good temporal resolution and is widely available—features congruous with the needs of the stem cell imager. The balance of this chapter focuses on the use of ultrasound in stem cell tracking and describes the basis of contrast in ultrasound imaging as well as the types of ultrasound labels, examples of direct and indirect labeling with ultrasound, and some perspectives on future growth in the field.
2 The Rationale for Ultrasound Imaging
Ultrasound imaging offers many advantages that are useful to studying stem cell therapy . First, ultrasound is very accessible and affordable. It is by far the most common piece of imaging equipment worldwide from small rural clinics to major research university hospitals. Second, ultrasound offers spatial resolution advantages (~50 μm) that are useful to identify subtle differences in treated tissue. Third, ultrasound offers excellent temporal resolution (up to 1000 frames per second)—this is critical for instantaneous knowledge of the cell location and the cell number. Fourth, ultrasound data can be quantitative, which is critical for identifying not only the presence of the cells, but also their number. Fifth, ultrasound offers a broad portfolio of complementary imaging sequences that can be used to enhance the cell data. That is, the ultrasound can collect imaging data about the surrounding anatomy and tissue behavior that complements the functional information from the cells. This includes motion mode (M-mode) imaging that studies repetitive motion such as the heart chamber [16], Doppler imaging which monitors the direction of movement [17], and various quantification schemes that can be used to estimate organ size or cardiac behavior including the left ventricle ejection fraction [18]. However, ultrasound images can also be very difficult to interpret due to the high background noise and/or complicated acoustic properties of different tissues. This is especially problematic in stem cell imaging—thus, it is critical to use either a direct or an indirect imaging technique to increase the cell-specific contrast.
The features of ultrasound and a comparison to other techniques used for stem cell tracking are shown in Fig. 2. Ultrasound is particularly powerful because of its high temporal resolution. This allows nearly instantaneous readout of the features of interest including the cell location and cell number. On the y-axis in Fig. 2, we plot the spatial resolution—how fine of an image can be created or the smallest distance between two objects that can be resolved. In ultrasound the spatial resolution is a function of the frequency used to create the images. At 70–100 MHz, the resolution can be as high as tens of micrometers, while at clinical frequencies (2–10 MHz), the spatial resolution is much lower—hundreds of microns to millimeters. As a trade-off, lower frequencies do offer better penetration through tissue—clinical frequencies can easily penetrate up to 25 cm into human beings depending on the tissue type, while higher preclinical frequencies (used in rodent models of human disease) are often limited to 2–3 cm of tissue. Importantly, the temporal resolution (frames per second) does not change as a function of frequency.


Fig. 2
Performance features of various imaging modalities. The temporal resolution (time between images) and spatial resolution (distance between points that can be distinguished) are plotted for different imaging modalities. Ultrasound offers good temporal and spatial resolution
2.1 Ultrasound Mechanism and Types of Ultrasound
Ultrasound imaging in vivo is not entirely different than the approach used by bats to “see in the dark.” Ultrasound imaging uses a tool called a transducer (Fig. 3a). The transducer is simply a tool that both emits and receives ultrasound pressure waves. As the emitted sound wave passes through tissue it is scattered and reflected (echoed) back to the transducer. The image is created by interpreting the backscattered sound and direction of sound as well as the speed of sound, the time of emission, the time of arrival back at the transducer, and the angle of return. The reconstructed image thus reports the distance to the object, the size of the object, and its density (or impedance mismatch with the surrounding tissue). Most ultrasound images are two dimensions, but 3D ultrasound is possible by moving the transducer over the surface to be imaged (like a panorama shot on a mobile phone camera). Ultrasound can also be done in Doppler mode in which changes in the sound wave’s pitch and phase are used to gain even more information. This is analogous to the sound differences when a siren is moving towards you and away from you. This can be used to determine blood flow or study other movement events inside the body.


Fig. 3
Ultrasound mechanism. The mechanism of contrast in ultrasound. Traditional B-mode imaging (a) uses backscattered pressure waves to generate contrast. In photoacoustic imaging (b), an incident light is absorbed by the target tissue. Once absorbed the target heats and swells creating pressure differences that can be detected acoustically
In addition to Doppler ultrasound , photoacoustic ultrasound is another important subtype of ultrasound imaging. In the photoacoustic effect ultrasound waves are created due to incident light pulses on the tissue (Fig. 3b). That is, regular ultrasound is “sound in—sound out,” and photoacoustic imaging is “light in—sound out.” Alexander Graham Bell originally described the photoacoustic effect , but it has not been until recent years that people have used it for imaging because the transducers have become more sensitive and lasers have been developed with very short pulse lengths.
The mechanism is based on optical absorption—when the light is absorbed by the target tissue, the target heats, and swells. This thermal expansion then generates pressure waves that can be detected acoustically. The fundamental advantage of photoacoustic imaging is that it combines the high temporal and spatial resolution of ultrasound with the good contrast and spectral nature of optical imaging. Photoacoustic imaging can use both exogenous absorbers such as hemoglobin, deoxyhemoglobin, and melanin or artificial contrast agents targeted to site of interest or a cell of interest.
A third type of ultrasound imaging is contrast-enhanced ultrasound. This uses an exogenous agent such as a perfluorocarbon microbubble to artificially increase the ultrasound signal at the site of interest. Contrast-enhanced ultrasound can move beyond the anatomical images that are created with traditional imaging (bone, muscle, etc.) to imaging protein expression levels or specific stem cell types. In the following section, we discuss some of the types of ultrasound contrast agents used as well as their applications in stem cell therapy . Because of the performance features of ultrasound it is somewhat surprising that there are relatively few reports of this technology in the literature. We will present these case studies in chronological order and highlight various examples of direct and indirect imaging via ultrasound .
2.2 Ultrasound Imaging of Cardiac Stem Cell Therapy
Heart disease is the most common cause of death in the developed world. Stem cell therapy has shown encouraging initial results in treating heart disease [4], and yet is plagued by poor long-term efficacy because of poor cell viability after implantation [5, 19]. This is attributed to two fundamental challenges—(1) ischemia and inflammation in the treated area [19], and (2) mis-injection or implant into highly fibrotic tissue [20, 21]. Because of these barriers, one example showed that fewer than 2 % of implanted dendritic cells remain viable after 4–8 weeks [21] with cells mis-injected in 50 % of patients [22]. Ultrasound is ideally suited to solve these challenges with poor delivery. Physicians already use ultrasound to image the catheter used for delivery of cells into the cardiac tissue, but the cells have a low impedance mismatch with the surrounding tissue and thus have low contrast.
Ultrasound is particularly attractive in cardiac stem cell therapy of the heart because it can be combined with the established use of echocardiography. Echocardiography is also known as a “cardiac echo” or just an “echo,” and it is used in the diagnosis and prevention of disease. Output parameters from echocardiography include the size and the shape of the ventricles and the left ventricle ejection fraction, which allows physicians to see how the heart chambers work in synchrony. This can be complemented by Doppler imaging to understand blood flow rates and directions.
In 2005, Rodriguez-Porcel and coworkers showed that ultrasound has fundamental utility in stem cell therapy [20]. Whereas prior work up to this point had performed open chest injection into the cardiac muscle, this study used ultrasound guidance in the parasternal long-axis view to deliver cells with a 28-gauge catheter. The cells were stably transfected cardiomyoblasts (plasmid–cytomegalovirus–firefly luciferase), and the target site was the anterior cardiac wall. The advantage of this approach is that the surgery is much less invasive and the surgeon can see the location of the catheter in real time. These researchers performed this work in 11 rats and then confirmed cell delivery with the bioluminescence reporter embedded in the cells. The bioluminescent signal was positively correlated with the number of cells transplanted (R 2 = 0.94, P = 0.03).
One limitation of this work was the poor ultrasound signal from the cells. That is, the investigators could only know the location and number of the cells with downstream analysis via bioluminescence. Thus, in 2006 Bara and colleagues [23] used ultrasound not only to image the injection but also to image the cells (Fig. 4) [23]. Here, cells were labeled with a clinically approved formulation of iron oxide (CliniMACS). These materials are used for magnetic based cell separation and consisted of a 50 nm iron oxide core coated with a dextran shell that is finally annealed to a monoclonal antibody specific to the target of interest. The authors selected CD133 because it is a marker of hematopoietic stem cells. After purifying CD133+ stem cells from bone marrow aspirates, cell identify and purity were confirmed with CD34 flow cytometry.


Fig. 4
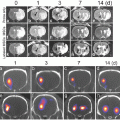
Direct imaging with magnetic particles. Swine hearts are imaged at various stages of stem cell therapy . In each image, the dark oval in the center is the left ventricle. Note the clear boundary between the interior and exterior of the heart wall in c (between red and green arrows). Panel a shows injection of unlabeled cells in the area highlighted with red circle. Little signal increase is seen. When the nanoparticle contrast agent alone is injected, hyperechoic regions are seen (b). Panels c and d are pre- (c) and post- (d) injection images of animals treated with five million nanoparticle-labeled stem cells. Reproduced with permission from Ref. [25]
These cells were delivered to swine models of human ischemia created via ligation/reperfusion [24]. The swine received either stem cells (5 million) or a sham injection of saline. Imaging used transesophageal echocardiography , which is a specialized type of ultrasound imaging that places a transducer in the throat of the subject to increase resolution versus ultrasound imaging via the chest wall. Figure 4 presents data including cells without any label (Fig. 4a), the ClinicMACS particles alone (Fig. 4b), as well as an animal before (Fig. 4c) and after treatment with labeled cells (Fig. 4d). The hypoechoic (bright) areas in Fig. 4b, d correspond to the increased sound backscatter due to the particles. The authors showed that the labeled cells had more signal than unlabeled cells but had challenges with long-term cell tracking. They concluded that this technique was perhaps best suited for the delivery event and that a complementary technique might be superior for long-term tracking.
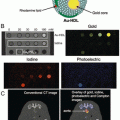
In 2013, Jokerst et al. extended this to multimodal imaging with MRI and ultrasound . In contrast to the magnetic nanoparticles used by Bara [23], this group used silica nanoparticles as ultrasound contrast agents [25, 26]. These have a key advantage in that the nanoparticles can be loaded inside the stem cells whereas the magnetic particles were on the cell exterior. This is important because contrast agents on the cell exterior can easily become detached in vivo and lead to erroneous signal. The silica nanoparticles are also triple-modality agents—ultrasound signal is generated via impedance mismatch of the silica, optical imaging is enabled by an embedded fluorophore, and T1-weighted imaging is possible via chelated gadolinium (Fig. 5) [27]. Stem cells could then be labeled with these materials for both instant imaging at the time of delivery via ultrasound and long-term follow-up with MRI.


Fig. 5
Direct imaging with nanoparticles . Ultrasound (a) and MRI (b) images were collected before injection of 500,000 human mesenchymal stem cells in a rodent model. After injection, the animals were imaged again with obvious increases in signal indicating the presence of the cells on ultrasound (c) and MRI (d). Reproduced with permission from Ref. [29]
Once labeled, the nanoparticles increased the ultrasound and MRI contrast of labeled human mesenchymal stem cells 700 % and 200 %, respectively. The authors investigated the behavior of the agent on cell metabolic activity, proliferation, or pluripotency, but did not find any significant change. Electron microscopy and ultrasound imaging suggest that the mechanism of action is in vivo aggregation of the 300 nm silica nanoparticles into larger silica frameworks that amplify the ultrasound backscatter. Detection limits in cardiac tissue were 250,000 cells via MRI and 70,000 via ultrasound with cell imaging possible in animal models for 13 days after implantation (Fig. 5). This ultrasound-guided cell delivery and multimodal optical/ultrasound/MRI intracardiac cell-tracking platform could improve cell therapy in the clinic by minimizing mis-delivery or implantation into fibrotic tissue . Moreover, these materials were compatible with both preclinical and clinical ultrasound frequencies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree