Chapter 27 Functional magnetic resonance imaging (fMRI) is based on the blood oxygen level–dependent (BOLD) contrast effect and neuronal activity–cerebrovascular flow coupling. Underlying the BOLD effect is the ability of MRI to differentiate magnetic properties of hemoglobin oxygenation states. Oxygenated blood (oxyhemoglobin) is diamagnetic, producing little susceptibility-related dephasing on MR signal. Deoxyhemoglobin is paramagnetic and elicits a more prominent effect on local field homogeneity and phase coherence, resulting in signal loss. Changes in the relative concentrations of oxyhemoglobin and deoxyhemoglobin in the vascular bed therefore result in changes in regional MR signal. The increase in cerebral blood flow that accompanies neuronal activity results in a relative increase in oxyhemoglobin concentration and a resultant localized increase in MR signal.1 Although seemingly straightforward, the actual physiology underlying the BOLD response is complex and is the subject of ongoing research.2 Many anatomic and physiologic changes occur during brain development that have the potential to alter the BOLD response in children compared with adults.3,4–6 Despite these physiologic differences, the basic BOLD response in children is generally similar to that in adults,3,7 albeit with some task-related differences.8 Neonates and infants may exhibit significantly different BOLD responses than do older children and adults, complicating interpretation in this age group.9–11 The requirements for fMRI performance include an MRI scanner with gradient hardware capable of performing fMRI useable sequences, stimulation/presentation hardware and software linked to the scanner to allow for synchronization of stimuli and MR imaging, hardware and software for documenting patient responses, and postprocessing software for producing activation maps. The small BOLD effect changes in MR signal typically are detected by echo planar imaging (EPI) T2*-sensitive gradient recalled echo techniques. Because of increased signal to noise and sensitivity for BOLD contrast effects, 3T scanners are preferred for fMRI studies.12,13 Performance of useful clinical fMRI examinations in children requires specialized preparation and resources. Before the scheduled examination, the patient is assessed for underlying neurologic deficits, developmental level, and ability to complete the fMRI paradigms. Explanation of the MR procedure and fMRI paradigms to the patient and parents in a calm, child-centered environment is critical. Practicing the fMRI paradigms is important to maximize performance and to adapt the tasks for the patient’s clinical and developmental level. Video presentations and mock scanners are highly useful in preparing children for the fMRI environment.14,15 Patient comfort should be maximized. Patient motion can influence fMRI performance in children.14,16 Despite some ability to retrospectively correct for head motion, gross head movement typically results in unusable fMRI data. Head coil bite bars, inflatable head cushions, and forehead and chin straps may be used but can be difficult to implement. Head motion is more pronounced in younger children and in boys.16 Older children and girls have a higher rate of successfully completed examinations.14 With care and adequate preparation, most children presenting for clinical fMRI studies can complete fMRI examinations with multiple administered paradigms. The routine application of real-time fMRI processing can reduce the number of inadequate studies.17 Repeated samplings of the brain (one brain volume scan during each repetition time period) while the subject alternates between active cognitive and control tasks typically is performed (the fMRI “paradigm”). Typical fMRI paradigms require 3 to 7 minutes of imaging time for acquisition of 100 or more image volumes during three to five cycles of alternating behavior. Although many approaches are possible, clinical fMRI is most commonly performed in a “blocked-periodic” design in which blocks of task and control (baseline) conditions are administered sequentially.18 The fMRI paradigm that is used ideally will result in activation of brain regions involved with the sensory, motor, or cognitive task presented, without activation in other regions. The proper choice of control and task conditions is important to allow this distinction and must be carefully matched to elicit detectable BOLD signal and isolate the function of interest.19,20 For successful performance of fMRI examinations in children, utilization of age and developmentally appropriate paradigms is mandatory.14,21 Imaging processing is required for fMRI and should involve the interpreting radiologist. After acquisition of images during the fMRI paradigm, the images typically are processed to diminish EPI artifacts, correct for susceptibility-related distortions, limit effects from patient movement during the paradigm, align and transform the T2* EPI images to a higher resolution anatomic dataset, and statistically analyze the images for BOLD signal changes between the task conditions on a voxel by voxel basis (the statistical map) (Fig. 27-1).22 Increasingly, streamlined, clinically oriented options are being offered on most clinical MR systems or by a growing number of third-party vendors. The most common statistical tests used for clinical fMRI are the general linear model21 and the cross-correlation method.18 Determination of the optimum statistical threshold for use in individual clinical patients is a complex issue.22,23 Evaluating fMRI studies at multiple different thresholds is important to maximize clinical effectiveness. Figure 27-1 Example of bilateral finger tapping functional magnetic resonance imaging (fMRI) and the effect of smoothing on the appearance of fMRI statistical maps. Some fundamental concepts must be kept in mind when performing and interpreting fMRI studies in clinical patients.24 For example, fMRI activation regions are not functionally specific, and lack of activation in a brain region does not indicate lack of critical brain function. The fMRI procedure is an indirect evaluation of neuronal function and relies on statistical mapping techniques that are not clinically standardized. The BOLD effect can be directly altered by pathologic states with changes in cerebrovascular autoregulation and neurovascular coupling,25 including vascular steno-occlusion, tumors with high vascularity, and arteriovenous malformations.25–27 Artifacts from regions of susceptibility effect (e.g., skull base, sinuses, hemorrhage, and prior surgery) may limit fMRI sensitivity. Sedation also can alter the BOLD response significantly.28 The most common and reproducible application of fMRI in clinical patients is assessment of the sensorimotor system (Fig. 27-2).24,29 The fMRI activation areas are somatotopically arranged along the central sulcus. Secondary regions including the supplementary motor area and premotor cortex often commonly are identified. Validation with direct electrocortical simulation (ECoS), the surgical gold standard, generally has been excellent,30–33 with recent studies demonstrating nearly 100% concordance.29 Sensorimotor fMRI evaluation is most useful when the normal anatomic relationships relating to the central sulcus are distorted by adjacent mass lesions; fMRI can help guide operative approaches in these cases (Fig. 27-3). High success rates of motor fMRI (93%) in children undergoing surgery for epilepsy have been documented.34 Figure 27-2 Examples of different motor paradigms useful for clinical functional magnetic resonance imaging. Figure 27-3 A 15-year-old boy with progressive right-sided motor weakness. Multiple clinical and fMRI studies have established that a left hemispheric dominance exists for semantic and phonological language functions in most persons. Most fMRI studies of language lateralization in children have shown similar patterns of activation compared with adults, supporting the theory that language networks are established by early childhood. However, multiple cross-sectional and longitudinal fMRI studies have demonstrated changes in BOLD localization20,35–39 with multiple language paradigms during development. In general, greater and more widespread activation is present in children compared with adults, which likely is related to differential brain maturation. Hemispheric language dominance is related to handedness, with approximately 95% of right-handed subjects being left hemispheric dominant for language, whereas approximately 20% of non–right-handed subjects (those who are ambidextrous or left handed) exhibit atypical (nonlateralizing or right-sided) hemispheric language dominance.40 These findings should be kept in mind when interpreting language fMRI examinations in children. Multiple fMRI paradigms have been created to assess different aspects of language function (Fig. 27-4 and e-Fig. 27-5). It is important to utilize multiple language tasks in clinical patients to more fully define language processing.20,41–43 The use of multiple tasks reduces the likelihood of nondiagnostic findings, improves inter-rater reliability, and helps in the confirmation of language laterality.43 Three of the most studied paradigms in children are verb generation, semantic decision, and story processing (Fig. 27-2). Figure 27-4 Examples of language functional magnetic resonance imaging (fMRI) paradigms in a 12-year-old right-handed girl with intractable epilepsy.
Functional Magnetic Resonance Imaging
Physiologic Basis of Functional Magnetic Resonance Imaging
Technical Considerations for fMRI Performance in Children
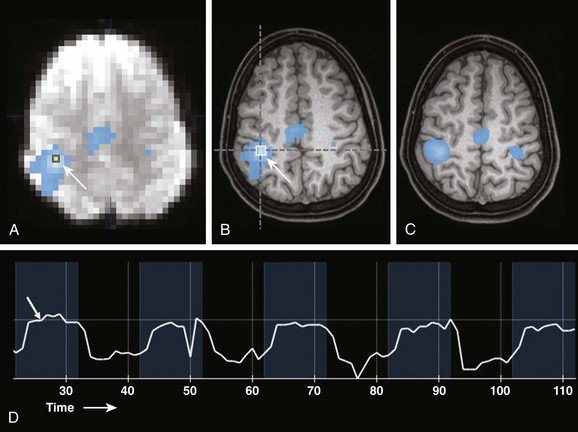
A, A source echo planar image with an overlayed nonsmoothed statistical activation map. A single voxel region of interest (arrow) is located in the region of maximal statistical significance. B, A nonsmoothed fMRI statistical activation map overlayed onto a 1 mm isotropic T1-weighted image shows activation overlying the right central sulcus in the hand motor region (arrow). Overlay onto anatomic MRI images allows the spatial relationships of the fMRI activation areas to be appreciated and related to anatomy and pathology. C, An fMRI statistical activation map overlayed on a 1 mm isotropic T1-weighted image with smoothing applied to fMRI data. D, Actual signal intensity within a voxel of interest (arrow in A) during the fMRI paradigm. Blue time periods are during finger tapping. Black periods are times of rest. Note the delay in the signal increase (hemodynamic response, arrow) after task initiation.
Clinical Applications of fMRI in Children
Limitations
Sensorimotor System Evaluation
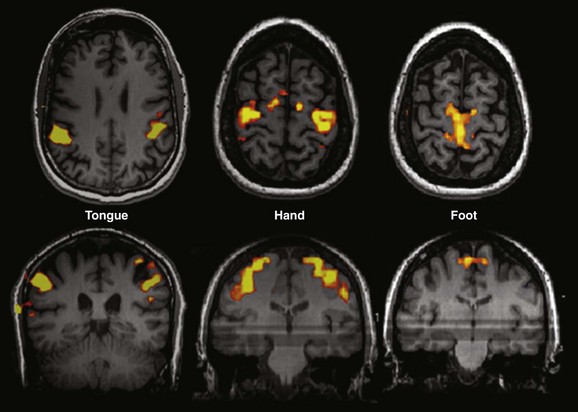
Tongue: Sequential tongue movement while the mouth is closed. Hand: Sequential bilateral finger tapping. Foot: Sequential bilateral foot flexion and extension. Typically performed clinical paradigms include sequential finger thumb opposition, hand grasping, wrist flexion and extension, foot flexion and extension, lip puckering, and tongue movement for motor strip assessment and tactile stimulation with brushes or air puffs for sensory component evaluation.
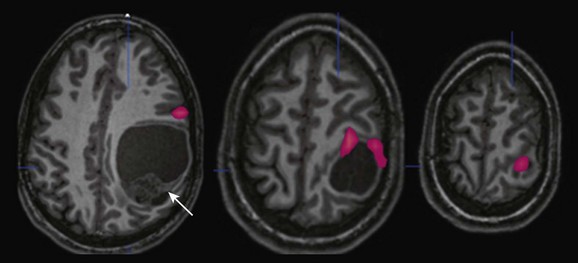
Anatomic magnetic resonance imaging revealed a large cystic and solid mass within the left parietal lobe (arrow) that was markedly distorting normal anatomy; functional magnetic resonance imaging (fMRI) was requested to outline the location of the motor strip more definitively. An fMRI image obtained during right-sided sequential finger tapping demonstrates activation along the superior and anterior aspects of the mass. A posterior surgical approach was performed with gross total resection and no new deficit. Pathology revealed that the mass was an anaplastic ependymoma.
Language Evaluation
Paradigms for Language Assessment
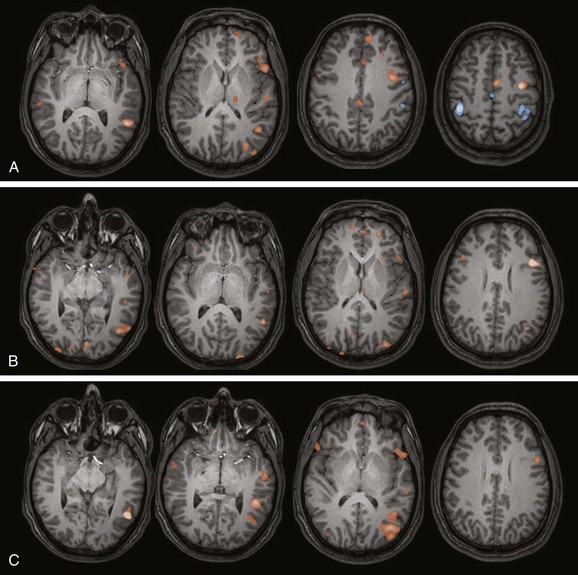
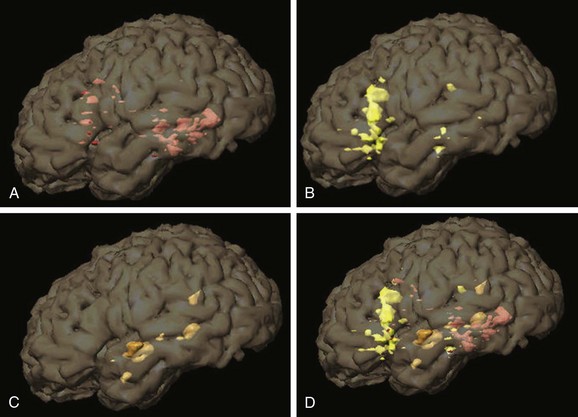
A, Verb generation (control task: finger tapping). A statistical map of fMRI activation during verb generation (orange areas) demonstrates left lateralization, particularly in the left inferior frontal lobe and left temporal lobe, which is typical for this paradigm. Activation during finger tapping is noted along both central sulci in the hand motor regions, as well as the putative supplementary motor area in the midline. B, Semantic decision (control task: tone discrimination). A similar left lateralizing pattern is noted with robust inferior frontal and temporal parietal activation, which is typical for this paradigm. The semantic decision task provides an additional assessment of frontal and temporal language areas and has the added capability of allowing direct assessment of patient performance. C, Story processing (control task: backwards speech). Clear left lateralizing activation is noted in the temporal lobe. Left lateralizing inferior frontal activation also is noted. These paradigms together indicate typical left lateralization of language in this patient.