Fig. 1
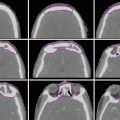
Target delineation of GTV1 (red), CTV1 (aqua), GTV2 (green), and CTV2 (purple) in a patient with a left frontotemporal GBM (post-biopsy only) on axial MRI FLAIR sequence (left), CT (middle), and T1 contrast-enhanced MRI (right) slices. In this case, there was significant edema, resulting in a dramatic difference in volumes between GTV1 and GTV2, as well as CTV1 and CTV2
Low-grade gliomas (LGGs) demonstrate a propensity for progression to a higher grade over time, possibly through accumulation of additional genetic events. Malignant transformation can occur in as many as 80 % of low-grade diffuse astrocytomas (Gondi et al. 2013).
An adequate understanding of intracranial anatomy is essential when approaching these tumors. Accurate assessment of location of gliomas and proximity to critical structures is crucial in determining the appropriate degree of surgical intervention. These factors must also be taken into account when designing a target for radiation therapy.
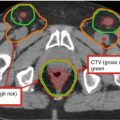
Pertinent anatomic structures to delineate in a patient with a glioma include (Fig. 2):




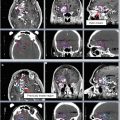
Fig. 2
(a) Sagittal CT showing the chiasm (purple) and brainstem (light orange) contours. (b) CT scan on bone windows facilitates identification of the cochleae. (c) Bilateral lenses (yellow & pink), eyes (teal & lavender), lacrimal glands (orange & green), and brainstem (light orange) outlined on axial CT. (d) Axial CT of pituitary gland (green). (e) Sagittal CT of the pituitary gland (green). (f) Axial CT of hippocampus (orange). (g) Sagittal of hippocampus (orange). Hippocampi are best contoured on MRI and transposed to the CT images
Lenses
Eyes (retina)
Lacrimal glands
Optic nerves
Optic chiasm
Hippocampus (not routinely done)
Pituitary gland
Cochleae
Brainstem and upper cervical spinal cord (as appropriate)
Specific cranial nerves (in select circumstances)
2 Diagnostic Workup Relevant for Target Volume Delineation
Prior to surgery, it can be helpful to obtain functional MR imaging to determine potential injury that could result from aggressive tumor resection, based on location. Intraoperative monitoring is often employed to further decrease the risk of adversely affecting function.
Following surgical intervention, immediate (within 48 h) postoperative MR imaging with contrast is strongly recommended. Thin slices (e.g., 1–2 mm) are preferred for enhanced image resolution and more accurate fusion with the planning CT images, which should ideally match the slice thickness of the MR images.
Radiographic imaging is helpful in treatment planning but almost universally underestimates the true extent of disease.
In 35 glioblastoma multiforme (GBM) patients, 78 % of recurrences were found to be within 2 cm of the tumor as visualized on CT at autopsy in a study by Hochberg and Pruitt (1980). This pattern was validated by Wallner et al. (1989). These data are the basis for the definition of the boost gross tumor volume (GTV), treated to higher doses (e.g., 60 Gy).
In a study by Kelly et al. (1987), isolated tumor cells were noted to extend to cover T2 changes and beyond on MR imaging. This was confirmed with serial stereotactic biopsies. These data are the foundation for the definition of the initial GTV, treated to lower doses (e.g., 46 Gy).
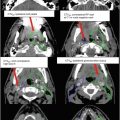
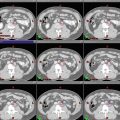
For patients with extensive edema and only a small volume of enhancing tumor, a diagnostic MR image at mid-treatment is recommended. In such cases, emergence of further areas of enhancement can be seen, requiring expansion of the boost (GTV2) volume to cover these areas. Figures 3, 4, and 5 include images that show multifocal edema and a small unifocal enhancing tumor. It is possible that enhancing disease may develop in other areas of edema over a span of weeks.


Fig. 3
(a) Axial FLAIR and CT images of a patient with multifocal GBM demonstrating superior sections of GTV1 (red), CTV1 (aqua) and CTV2 (purple). A 1.5 cm expansion, rather than 2 cm, was used to create both CTVs given the expansive FLAIR changes and concern for volume of normal brain being irradiated. (b) Axial FLAIR, CT and TI contrast-enhanced images of the same patient with a multifocal GBM. Contoured structures include GTV1 (red), CTV1 (aqua), GTV2 (green) and CTV2 (purple)

Fig. 4
Axial MRI FLAIR and CT views of the same patient with multifocal GBM demonstrating inferior portion of GTV1 (red), CTV1 (aqua), and CTV2 (purple)

Fig. 5
Sagittal CT views of the same patient with multifocal GBM demonstrating GTV1 (red), CTV1 (aqua), GTV2 (green), and CTV2 (purple)
MR fluid-attenuated inversion recovery (FLAIR), T1, and T2 (if significantly different from FLAIR) sequences should be fused with the simulation CT scan for contouring guidance.
In situations in which cranial nerve abutment is a concern, a 3D fast imaging employing steady-state acquisition (FIESTA) MR series can be helpful in visualizing and sparing the nerves. A 3D spoiled gradient recalled (SPGR) MR image can facilitate contouring of the hippocampus.
For patients with recurrent GBM, the addition of PET/CT imaging should be considered. Because it is difficult to distinguish between postradiation changes and disease recurrence on MR imaging, PET occasionally can be helpful. In these instances, PET/CT should be fused to the tumor on simulation CT and to MR imaging. Amino acid PET imaging for this purpose is frequently used in Europe but is not approved for this use in the United States.
3 Simulation and Daily Localization
In the majority of situations, patients should undergo CT simulation supine with the head in a neutral position. Intravenous contrast is not recommended.
The head and neck must be immobilized, preferably up to the shoulders, using a custom mold or mask to increase setup accuracy. Bite blocks can be useful in those situations in which angular rotations are a problem (in our practice, these are used infrequently). One specific advantage of a bite block is the possibility of incorporating into it markers that can be tracked by cameras, so that intrafraction motion can be monitored. This would be useful in fractionated stereotactic approaches.
Thin-slice CT imaging is recommended (at 1–2 mm intervals) and, for ease of fusion, ideally should match the MR image slice thickness.
Multimodal MR fusion, using at least the contrast-enhanced T1 and FLAIR sequences, should be fused to the planning CT. Although the entire anatomic volume should be matched, the primary matching focus is the tumor. PET/CT fusion can be helpful in select situations, as mentioned above.
4 Target Volume Delineation and Treatment Planning
Standards for treatment of HGGs are directed by data from three seminal studies from the 1980s that highlighted the extent of microscopic disease well beyond enhancing tumors or radiographic changes (Hochberg and Pruitt 1980; Wallner et al. 1989; Kelly et al. 1987). This finding drives the principles of contour generation in these cases.
Because the infiltrative nature of HGGs results in large fields, the GTV is divided into two components: one that incorporates all (or almost all) possible malignancy and one that focuses on a region of known or gross disease or the region at highest risk of developing recurrence.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree