Fig. 22.1
Observed survival rates for 15,070 cases with carcinoma of the cervix uterus. Data from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) diagnosed in years 2000–2002. Stage 0 includes 7,119 patients; Stage IA, 1,530; Stage IB, 2,249; Stage IIA 453; Stage IIB, 1,518; Stage IIIA, 191; Stage IIIB, 1,009; Stage IVA, 213; and Stage IVB, 788. (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC. http://www.springer.com)
Cervical cancer is associated with risk factors, such as low socioeconomic class, diet, chlamydia and HIV infection, geography, increased sexual activity, number of pregnancies and age at first pregnancy, cigarette smoking, use of oral contraceptives or diethylstilbestrol (DES), and human papillomavirus. The most important risk factor for cervical cancer is infection by the human papillomavirus (HPV). High-risk viral types are HPV 16, HPV 18, HPV 31, HPV 33, and HPV 45, about two-thirds of all cervical cancers being associated with HPV 16 and 18 [2]. Vaccines have been developed to help prevent infection with some types of HPV; currently, two HPV vaccines have been approved for use in the United States by the Food and Drug Administration (FDA) [2].
Pathophysiology and Clinical Presentation
There are generally no symptoms in women with precancerous lesions and early cervical lesions. Abnormal vaginal bleeding including postmenopausal bleeding, postcoital bleeding, intermenstrual bleeding, menorrhagia and spotting between periods, abnormal discharge, painful coitus, and bleeding after vaginal examination are some of the symptoms associated with advanced stages and invasive cancers. In some advanced stage cases, symptoms of bowel obstruction or urinary tract obstruction may be seen at presentation [3].
Cervical intraepithelial neoplasia (CIN) is a precursor lesion of cervical cancer and is graded based on cellular dysplasia into three groups: CIN 1 with minor dysplasia, CIN 2 with moderate dysplasia, and CIN 3 or carcinoma in situ with severe dysplasia. Up to 40% of CIN 3 lesions could develop into invasive cervical cancer if left untreated.
Histologically, 80–90% of all cervical malignancies are squamous cell carcinoma (SCC) (Table 22.1), with 25% well-differentiated, keratinizing, large-cell SCC histology, while 70% are moderately differentiated, nonkeratinizing, large-cell SCC. Small-cell undifferentiated carcinoma forms about 5% of cases only but is associated with poor prognosis. Adenocarcinomas arise from endocervical-type cells and constitute 5–20% of all cervical malignancies. The histologic patterns of adenocarcinoma include well-differentiated mucinous adenocarcinoma, papillary adenocarcinoma, and clear cell types. Poorly differentiated and aggressive histologic subtypes of cervical adenocarcinoma are associated with a poorer prognosis than SCC. Miscellaneous uncommon or rare cancers of the cervix include variants of SCC and adenocarcinomas, mixed carcinomas, small-cell carcinoma, sarcoma, lymphoma, melanoma, and metastatic tumors. Metastases arise most often from endometrial primary while others include the ovary, colon, and breast primaries [3, 4].
Table 22.1
Pathologic types of cancer of the cervix
1. Squamous cell carcinoma (a) Carcinoma in situ (CIN) (b) Microinvasive carcinoma (c) Invasive squamous cell carcinoma |
2. Variants of squamous cell carcinoma (a) Verrucous carcinoma (b) Papillary squamous and transitional carcinoma (c) Lymphoepithelioma-like carcinoma |
3. Adenocarcinoma (a) Early invasive (b) Mucinous adenocarcinoma • Endocervical variant • Intestinal type, signet ring, and colloid variants • Minimal deviation variant (adenoma malignum) • Well-differentiated villoglandular variant (c) Endometrioid adenocarcinoma (d) Clear cell adenocarcinoma (e) Serous adenocarcinoma (f) Mesonephric adenocarcinoma (g) Microcystic endocervical adenocarcinomas |
4. Other epithelial tumors (a) Adenosquamous carcinoma (b) Glassy cell carcinoma (c) Adenoid cystic carcinoma (d) Adenoid basal epithelioma (e) Neuroendocrine tumors (f) Carcinoid tumors |
5. Mixed epithelial and mesenchymal tumors (a) Mullerian adenosarcomas |
6. Other malignant tumors (a) Sarcomas (b) Secondary tumors |
Preinvasive lesions can be treated with electrocoagulation, cryotherapy, laser ablation, or local surgery, while invasive cancers require surgery, radiation or chemotherapy, or combination therapy. The survival for localized cancer is good, being 92% at 5 years; however, the survival rates are lower for those patients with advanced disease (88% at 1 year and 73% at 5 years, respectively) [5].
Diagnosis of Cervical Cancer
The Papanicolaou test (called the Pap test) is a very effective screening test that has led to detection of early-stage cervical cancer. The American Cancer Society recommends screening for cervical cancer start approximately 3 years after a woman begins having vaginal intercourse but no later than 21 years of age [2]. Women with certain risk factors, such as HIV infection or some form of immunodeficiency, may be screened more often. For those who are 30 or more years, after 3 normal test results in a row, screening should be performed every 2–3 years. Women 70 years and older who have had 3 or more consecutive normal Pap tests in the last 10 years may choose to stop cervical cancer screening. Screening after total hysterectomy is not necessary, unless the surgery was done for cervical cancer [2].
Patients with suspicious findings on Pap smear or patients with high-risk HPV strains could be further evaluated with colposcopy or colposcopy-directed biopsies of the suspicious areas and, if necessary, with conization to establish the diagnosis [2–5]. Those diagnosed with cervical cancer would undergo clinical staging with imaging and detailed pathology, followed by surgical staging.
Classification of Disease and Role of Imaging
Patients are staged clinically according to the International Federation of Gynecology and Obstetrics (FIGO) system (Table 22.2) [6]. Conventional imaging studies recommended for clinical (FIGO) staging include chest x-ray, barium enema, and intravenous urogram [6]. However, ultrasound (US), computed tomography (CT), magnetic resonance (MR) imaging, and positron emission tomography (PET) are increasingly used.
Table 22.2
TNM and FIGO classification of carcinoma of cervix
TNM (FIGO stage) categories | Description |
|---|---|
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis* | Carcinoma in situ (preinvasive carcinoma) |
T1 I | Cervical carcinoma confined to uterus (extension to corpus should be disregarded) |
T1a** (IA) | Invasive carcinoma diagnosed only by microscopy. Stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less. Vascular space involvement, venous or lymphatic, does not affect classification |
T1a1 (IA1) | Measured stromal invasion 3.0 mm or less in depth and 7.0 mm or less in horizontal spread |
T1a2 (IA2) | Measured stromal invasion more than 3.0 mm and not more than 5.0 mm with a horizontal spread 7.0 mm or less |
T1b (IB) | Clinically visible lesion confined to the cervix or microscopic lesion greater than T1a/IA2 |
T1b1 (IB1) | Clinically visible lesion 4.0 cm or less in greatest dimension |
T1b2 (IB2) | Clinically visible lesion more than 4.0 cm in greatest dimension |
T2 (II) | Cervical carcinoma invades beyond uterus but not to pelvic wall or to lower third of vagina |
T2a (IIA) | Tumor without parametrial invasion |
T2a1 (IIA1) | Clinically visible lesion 4.0 cm or less in greatest dimension |
T2a2 (IIA2) | Clinically visible lesion more than 4.0 cm in greatest dimension |
T2b (IIB) | Tumor with parametrial invasion |
T3 (III) | Tumor extends to pelvic wall and/or involves lower third of vagina, and/or causes hydronephrosis or nonfunctioning kidney |
T3a (IIIA) | Tumor involves lower third of vagina, no extension to pelvic wall |
T3b (IIIB) | Tumor extends to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney |
T4 (IVA) | Tumor invades mucosa of bladder or rectum and/or extends beyond true pelvis (bullous edema is not sufficient to classify a tumor as T4) |
Nodal stage | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastasis |
N1 (IIIB) | Regional lymph node metastasis |
Metastasis | |
M0 | No distant metastasis |
M1 (IVB) | Distant metastasis (including peritoneal spread, involvement of supraclavicular, mediastinal, or para-aortic lymph nodes, lung, liver, or bone) |
Surgical staging is generally done in advanced disease to assess pelvic and aortic lymph node involvement, critical for further treatment and follow-up. Establishing the local extent of the primary cervical cancer is very important, and while involvement of pelvic or para-aortic lymph nodes may not affect the International Federation of Gynecology and Obstetrics (FIGO) staging, it identifies patients with a worse prognosis [6–9]. Spread of cervical cancer occurs initially by direct extension to local structures and regional lymphatics, later disseminating hematogenously to distant organs, such as bone, brain, liver, and lung. Nodal metastasis occurs sequentially initially involving the pelvic lymph nodes followed by para-aortic and supraclavicular lymph nodes. In up to 17% of the cases scalene nodes are also involved. Scalene node involvement is seen more commonly in patients who have aortic node disease [7–9]. Bone metastasis occurs infrequently, and therefore bone scans are not routinely indicated and should be used only in patients with bone pain. For initial staging, imaging is critical for the local extent of disease and for detection of local and distant nodal metastatic disease. Abdominal pelvic CT scan provides high-resolution anatomic detail and is an integral part of routine staging. Disease in the cervix, uterus, parametrium, adnexa, and pelvic as well as abdominal nodal involvement and extension of disease to extrapelvic sites can be accomplished with high sensitivity [10]. Evaluation of microscopic disease, however, is limited since the anatomic changes are subtle thereby reducing the sensitivity of CT, resulting in understaging. MRI has superior tissue characterization and is preferred for estimation of local extent. MRI can detect endometrial involvement in about 84–96% of the cases versus 55–80% by CT scans and has a high negative predictive value (NPV) [10–13]. Para-aortic nodal metastasis can be detected with high specificity, although sensitivity is only 50% [13]. Staging using physical examination, colposcopy, lesion biopsy, and cystoscopy or sigmoidoscopy may understage up to 20–30% of the stage I and II cases and up to 40% of the stage III cases; conversely, it may overstage approximately 64% of patients with stage IIIB disease [8, 9, 14–16]. Accurate assessment of tumor size, especially in endocervical location, parametrial and pelvic sidewall invasion, as well as lymph node and distant metastases, is extremely important for prognosis. However, at many times this assessment is limited in routine conventional imaging, [9] and additional imaging with [18F]FDG PET/CT can provide critical information for management of disease.
[18F]FDG PET Imaging
PET/CT is useful to assess local extent and pelvic nodal involvement and detect distant metastases. This information is useful to plan surgery and/or for radiation therapy planning, as well as for assessing response to neoadjuvant therapy and for posttreatment identification of persistent/recurrent disease.
Primary Cervical Cancer
[18F]FDG localizes in cervical cancer due to high Glut-1 expression by the tumor cells; however, there is no established correlation of SUV or Glut-1 expression to initial grade of histologic differentiation and to FIGO staging. Higher Glut-1 expression is seen in recurrent or persistent disease than in metastatic lymph nodes [17].
Most primary cervical cancers concentrate [18F]FDG (Fig. 22.2); however, poorly differentiated tumors and squamous histology tumors are more [18F]FDG avid. Higher SUVmax is associated with increased risk of nodal involvement: SUVmax <5 had 24% nodal involvement compared to 59% for patients with SUV of 5–14 and 72% for those with SUV >14. Higher uptakes are seen in squamous cell cancer, with average SUV of 11.91 (range 2.50–50.39) versus nonsquamous tumors (average SUV 8.05, range 2.83–13.92) and poorly differentiated (SUVmax 12.23) versus well-differentiated tumors (SUVmax 8.58) [18].
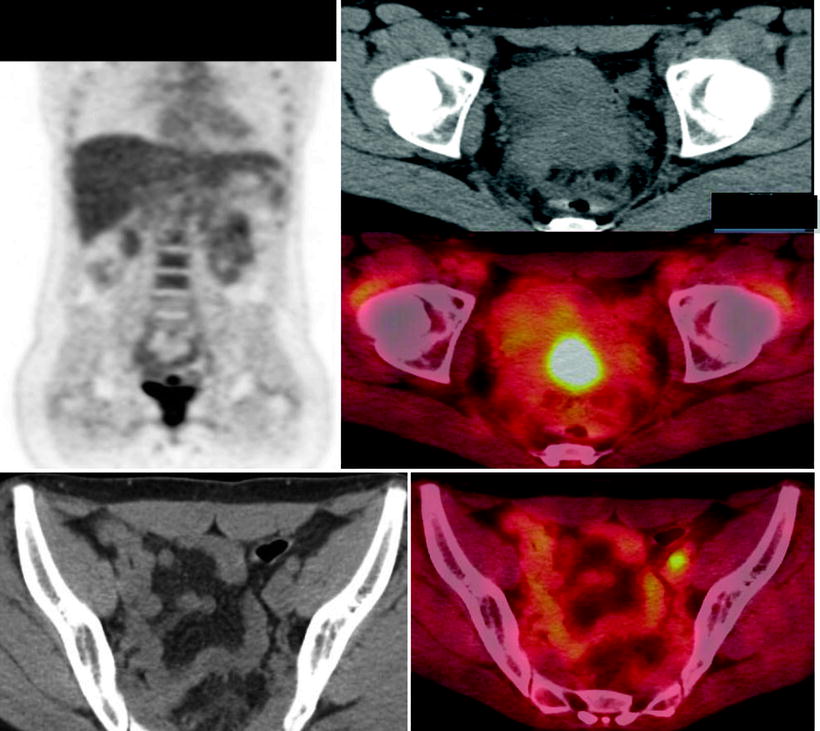

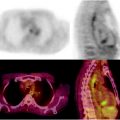
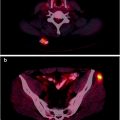
Fig. 22.2
(a, b) Primary carcinoma of the cervix: extent of disease at diagnosis. A 50-year-old woman with cervical cancer is shown. [18F]FDG PET/CT showed abnormal uptake (SUVmax, 13.6) in the cervix and uptake in left external iliac node
Early studies used dedicated PET scanners without CT capability. PET/CT scanners improve localization and hence diagnosis through a decrease in equivocal results. This is especially relevant for gynecologic malignancies, where peritoneal and serosal bowel implants may be obscured by physiologic activity. Evaluation with combined PET/CT imaging in gynecological malignancies has reported better results as compared to PET scan alone [19, 20].
In 13 primary and metastatic uterine cervical cancer patients, PET/CT allowed more definitive lesion localization in 30% and definite lesion characterization in 20% compared to PET alone. There was higher diagnostic accuracy with PET/CT than PET alone on a lesion-based analysis (92% vs. 78%), while no difference was noted for evaluation of metastatic disease [21].
[18F]FDG PET in Staging of Cervical Cancer
Clinical studies in small series of patients have shown the usefulness of PET/CT in staging cervical cancer, with sensitivities ranging from 38 to 86%. A definite advantage of [18F]FDG PET is the ability to detect involvement of lymph nodes that are benign by size criteria and to detect distant metastases that were not detected by other imaging modalities [22]. Perhaps the most critical use is in defining local pelvic and distant nodal status [23–25]. In a retrospective analysis (n = 41) [18F]FDG PET showed 100% accuracy in detecting disease for initial staging. For restaging, sensitivity was 82% and specificity 97% [21]. While MRI provides excellent evaluation of local tumor and parametrial involvement, PET is more useful for the evaluation of pelvic lymph nodes. An important caveat, however, is that microscopic disease is missed by both [26]. Due to larger scanning areas, estimation of thoracic or suprathoracic disease is possible in a single PET study. Tran and colleagues detected supraclavicular nodal involvement in about 8% of their patients, with a 100% positive predictive value (PPV) [22].
Sensitivity and accuracy are higher for advanced disease but low in early-stage disease including up to stages IA2–IIA [27–32], although [18F]FDG PET may detect abnormal lymph nodes better than CT [33]. Combined PET/CT is more accurate than side-by-side reading of CT and PET to detect involved nodes [34], though small-volume and microscopic disease may be missed by both. It has been shown that [18F]FDG PET is more sensitive than CT or MRI for detecting lymph node metastases in patients with cervical carcinoma [23, 24, 29, 31, 35, 36]. In a study of 22 patients with stage IB–IVA cervical cancer, Choi et al. found that PET/CT had a higher sensitivity (57%) than MRI (33%) for lymph node detection, nodal size being critical for detection; short-axis dimension of metastatic lymph nodes was 11.8 ± 3.5 mm, while that of nonmetastatic nodes was 6.5 ± 4.6 mm [28]. Understandably, the detection of lymph node metastases was higher in nodes with more extensive involvement. When compared to histopathology [25], [18F]FDG PET showed overall sensitivity of 37.8%, which increased considerably when the criterion for metastasis was restricted to tumor-invasion diameters of >5 mm (52.0%) and >10 mm (65.0%) [25]. The overall sensitivity of MRI for detecting lymph node metastases appears to be lower (32.0%) than PET [24, 25, 28, 30, 34], though some studies report high false negatives (8 [80%] out of 10 pelvic nodal metastases) in smaller lesions <10 mm in diameter. Overall sensitivities in different studies are widely variable, possibly due to differences in the completeness of surgical staging and the number of lymph nodes removed.
In a study of 120 patients with newly diagnosed cervical cancer with FIGO stage ≥IB, [18F]FDG PET/CT had 75% sensitivity and 96% specificity for nodal status in those who underwent surgery [32]. For para-aortic nodal disease, the PPV was 94%, NPV 100%, sensitivity 100%, and specificity 99%, while for distant metastasis PPV was 63%, NPV 100%, sensitivity 100%, and specificity 94%. False positives were seen in lymph nodes with granuloma and in reactive nodes in the axilla and groin. Distant metastases were seen in lymph nodes in the neck or mediastinum and metastases in the liver, lung, omentum, or bones. False-positive distant foci on PET were due to histiocytosis and inflammatory nodes. A single false-negative node was located in the paracervical region [31]. High predictive values of PET in detecting nodes, as shown in this study, may be useful in management; in particular, patients with PET-positive nodes can be offered radio-/chemotherapy instead of surgery, and patients with para-aortic nodal involvement can be offered extended fields for radiotherapy [31]. Micrometastasis detected only on histopathology is a frequent cause of false negative on the PET scan.
Many studies in early-stage disease report limited results for low-volume, especially micrometastatic, disease leading to higher false-negative rates [27, 30, 37–40]. In 120 patients with negative MRI for pelvic nodes, all PET-undetected nodes had micrometastasis with mean tumor focus of 4.0 × 3.0 mm (range 0.5 × 0.5 to 7 × 6 mm) [30]. Other groups have also reported false-negative lymph node rate between 13 and 32% of cases, though detection was overall better as compared to CT [38, 39].
PET in Locally Advanced and Advanced Cervical Cancer: Pelvic and Para-Aortic Nodal Assessment at Initial Staging
Early studies with 12 or more subjects and with clinical follow-up ≥6 months or histopathology as the reference standards showed a pooled sensitivity of 79% (95% CI, 65–90%) and pooled specificity of 99% (96–99%) for detection of pelvic or para-aortic lymph node metastasis in patients with newly diagnosed cervical cancer [23–26]. In these studies, PET was better than CT (47% sensitivity, 95% CI 21–73%) or MRI (72% sensitivity, 95% CI 53–87%) [29, 31].
In a study of 21 patients with stages IB to IVA cervical cancer, Sugawara et al. reported 86% sensitivity for pelvic and para-aortic lymph node metastasis, which was higher than CT sensitivity (57%) [23]. Rose et al. studied presurgical patients with stages IIB to IVA and reported a sensitivity of 75% and specificity of 92% in identifying PALN metastasis and a slightly higher sensitivity (100%) for pelvic node detection [23, 24].
In prospective studies, [18F]FDG PET imaging for PALN metastasis was compared with histopathology after para-aortic lymphadenectomy; the pooled sensitivity of PET for the detection of PALN metastasis was 84% (95% CI, 68–94%) and the pooled specificity was 95% (89–98%) [41 43–48]. Pooled data from more than 15 studies on [18F]FDG PET in cervical cancer report 84% sensitivity and 95% specificity for aortic node metastasis and 79% sensitivity with 99% specificity for pelvic node metastasis, respectively. In a meta-analysis for PALN detection [36], data from 10 studies in 385 patients showed an overall specificity of [18F]FDG PET as high as 97% (95% CI, 93–99%) but a low overall sensitivity that was highly variable among the studies (average 34%; 95% CI, 10–72%). In the 5 studies with prevalence of PALN metastasis >15%, estimated sensitivity and specificity were 73% (95% CI, 53–87%) and 93% (95% CI, 86–97%), respectively. With this diagnostic performance and assuming 15% prevalence, the calculated false-positive and false-negative rates were 35 and 5%, respectively. This suggests a higher value of PET in those patients with high probability of PALN metastasis than when used in those with low risk of PALN involvement [36]. [18F]FDG PET may be of value in those with negative CT or MRI of the abdomen [31, 45–47]. In a study with locally advanced cervical cancer patients who had negative conventional CT findings, PET/CT scans were done prospectively, followed by surgery. Based on histopathologic confirmation, the accuracy, sensitivity, specificity, PPV, and NPV of PET/CT for PALN metastasis were 75%, 50%, 83.3%, 50%, and 83.3%, respectively. Change of management based on PET/CT findings that occurred in 4/16 (25%) of patients [47]. The false-negative rate for PALN involvement detection by PET can vary between 4 and 17% [29, 32, 46, 47], most likely related to small lesions and micrometastasis. Using PET/CT only to determine radiation therapy fields in stage IB2/II cervical cancer could overlook a significant number of patients with histologic PALN involvement [29, 32, 47].
For pelvic nodal assessment, the use of [18F]FDG PET imaging for routine staging prior to radical hysterectomy and pelvic lymph node dissection is still controversial, though high specificity and NPV of [18F]FDG PET may be helpful in planning the appropriate surgical procedure [42] In a retrospective review, PET/CT was found to have limited sensitivity (36.4%) but high specificity for predicting pelvic lymph node metastasis (98.8%) [43]. Overall, PET is of value in nodal assessment. The clinical significance of the missed nodal involvement with microscopic disease, in light of the use of concurrent chemotherapy, is unclear.
Prognostic Value of PET/CT at Initial Staging
Nodal involvement detected by [18F]FDG PET in cervical cancer stratifies patients for recurrence and survival outcomes. Assessment of PALN metastasis is prognostically important and is related to progression-free survival in advanced cervical cancer. In those patients with negative CT, [18F]FDG PET has high sensitivity and is useful in detecting and localizing the distant disease [20, 21, 41, 42, 46, 48–57]. Presence of nodal disease on the [18F]FDG PET scan can predict cause-specific survival. Forty-seven patients with FIGO stage IIIB cervical cancer were evaluated before therapy; the 3-year cause-specific survival was 73% for those with no lymph node metastasis, 58% for those with only pelvic lymph node metastasis, 29% for those with pelvic and para-aortic lymph node metastases, and 0% for those with pelvic, para-aortic, and supraclavicular lymph node metastases [49]. Presurgical cervical tumor uptake of [18F]FDG is also linked to survival and recurrence. In 44 patients with FIGO clinical stage IB to IIA, SUVmax was compared with pathological prognostic factors after the initial treatment. The SUVmax was significantly higher in patients with deep stromal invasion, lymph-vascular space invasion, and tumor size of more than 4 cm. A reduced disease-free survival rate was seen in those with SUVmax greater than or equal to 13.4. The SUVmax of 13.4 or more was a significant independent predictor of recurrence, and high uptake may indicate the need for a more aggressive multimodal treatment [50].
Yen and colleagues found SUVmax in PALNs also to be a significant prognostic factor in patients with primary advanced squamous cervical cancer. In 70 patients with FIGO I to IV, SUVmax greater than or equal to 3.3 for PALN was a significant adverse factor [51]. Outcomes for patients following chemoradiation therapy were found to be best for those who did not have PALN on radiographic imaging by inspection at the time of surgery [52]. In multivariate analysis, detection of metastatic lymph nodes by imaging was associated independently with a poorer prognosis compared with patients who were identified as positive on surgery only [52].
In patients with locoregionally advanced cervical cancer, PET and MRI were compared with FIGO staging for impact on outcomes measured by overall survival, relapse-free survival, and time to failure, local failure, nodal failure, and distant failure. In 206 patients the overall survival rate was 59% at 5 years. Local failure was mostly associated with adenocarcinoma, while corpus involvement on MRI was significantly associated with nodal involvement. Tumor volume measured from MRI was an important predictor of locoregional relapse. Nodal status on PET was the major predictor of outcome in locally advanced cancer of the cervix treated with chemoradiation, superior to FIGO staging [53].
Evaluation of nodal disease by PET is also linked to prediction of cause-specific survival. In a study with 47 patients with FIGO stage IIIB cervical cancer evaluated before therapy [49], the 3-year cause-specific survival was highest for those with no nodes (73%) than for those with only pelvic lymph node metastasis (58%) or pelvic and para-aortic lymph node metastases (29%). Those with pelvic, para-aortic, and supraclavicular lymph node metastases had lowest survival. PET positivity for nodes is also the most important predictor for distant metastases [54].
Preoperative high uptake in the primary tumor is associated with recurrence [55, 56]. In 560 patients, for any given stage patients with PET-positive lymph nodes had significantly worse disease-specific survival than those with PET-negative lymph nodes. The hazard ratios for disease recurrence increased incrementally based on the most distant level of nodal disease, being lowest for pelvic followed by para-aortic and highest for supraclavicular nodes [55].
Distant nodal uptake has adverse prognosis. Involvement of the supraclavicular node or scalene nodes can be seen in about 0–17% of patients, more commonly in those who have aortic node disease [57, 58] with a PPV as high as 100% [57]. Therefore, PET findings might help in selecting appropriate patients for curative primary and/or salvage treatment [58].
[18F]FDG PET in Cervical Cancer: Impact on Management in Initial Treatment
[18F]FDG PET/CT can alter initial management by detecting nodal disease [42, 49]. Patients with cervical cancer whose CT and MRI imaging showed metastases limited to PALNs, inguinal lymph nodes, and/or supraclavicular lymph nodes were prospectively evaluated for impact on management with addition of PET or PET/CT. PET had positive clinical impact in 21 (44.7%) of the 47 patients, including detection of additional curable sites (n = 8), downstaging (n = 6), offering metabolic biopsy (n = 4), or change of treatment to palliation (n = 3) [59].
Accurate status of nodal involvement is critical for treatment planning. For those patients with PET-negative lymph nodes, there may be no incremental benefit of combined chemoradiation therapy versus surgical therapy alone [60].
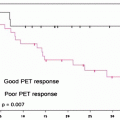
[18F]FDG PET/CT is useful to monitor neoadjuvant therapy [61–63]. In a retrospective study of 152 patients treated with external irradiation and intracavitary brachytherapy and concurrent cisplatin in most, the 5-year cause-specific survival estimate was 80% in those with no residual abnormality versus 32% in those with. Of those patients in whom progression of disease was seen, none were alive at 5 years [62].
[18F]FDG PET in Recurrent Cervical Cancer
[18F]FDG PET has high sensitivity in the detection of recurrent disease (Fig. 22.3). Overall sensitivity and specificity for detection of recurrence are reported to be as high as 86 and 94%, respectively [48, 64–67], while PPV and NPV were 85.7 and 86.7%, respectively [66]. Detection of small-volume disease is limited due to the lack of resolution; however, peritoneal disease can be detected when metabolically active [67].
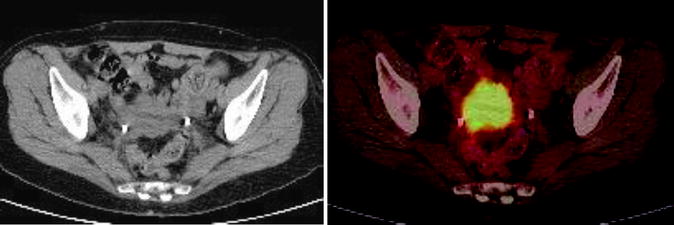

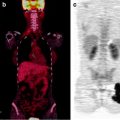
Fig. 22.3
A 32 year old woman carcinoma of the cervix Stage IIa. Treated with Chemoradiation therapy. She was referred for PET/CT evaluation. Increased [18F]FDG uptake is seen in cervix consistent with active disease
Combining PET with contrast-enhanced CT (CTce) improves lesion detection over low-dose CT (CTld). In 100 women with uterine cervical (n = 55) or endometrial cancer (n = 45), PET/CTce led to better delineation of equivocal lesions [68]. The sensitivity, specificity, and accuracy of PET/CTld were 90% (27/30), 97% (68/70), and 95% (95/100), as compared to those of PET/CTld of 83% (25/30), 94% (66/70), and 91% (91/100), respectively. Four equivocal regions by PET/CTld (local recurrence, pelvic LN metastasis, liver metastasis, and muscle metastasis) were correctly interpreted as positive by PET/CTld [67]. Sensitivity for detecting recurrence in asymptomatic women is also high with PET/CT, though less than that for symptomatic women (80% vs. 100%) [69].
Management of patients with elevation of the serum squamous cell carcinoma antigen (SCC-Ag) levels after complete response to prior treatment, in the absence of detectable disease using conventional imaging, is a clinical challenge. In these patients additional imaging with [18F]FDG PET can help to detect disease and guide management. Chang et al. reported detection of disease in 94% of patients with negative conventional imaging. This helped in early detection and therefore more appropriate therapy, leading to positive effect on survival in comparison to the historical data [70–71]. Yen and colleagues suggested a scoring system including primary radiation therapy, SCC-Ag level >4 ng/mL, and presence of symptoms to identify patients who would benefit from [18F]FDG PET imaging [72]. These data need to be validated in larger studies.
Surveillance [18F]FDG PET can detect asymptomatic recurrent disease that is potentially amenable to salvage therapy. A prospective registry study included 103 patients treated with definitive chemoradiation for advanced cervical cancer who demonstrated a complete metabolic response on their 3-month posttherapy [18F]FDG PET; 9/78 asymptomatic patients had tumor recurrence detected by PET, with isolated locoregional disease in 8 cases and distant disease in 1. The 3-year cause-specific survival was 59% for asymptomatic recurrences [73]. In another small study with 30 patients, PET was able to detect recurrent disease in 44% of asymptomatic patients [74].
In patients treated with radiation, [18F]FDG PET can help in early detection of recurrent disease or help confirm disease in those with abnormal serum tumor marker values. In such patients, sensitivity for detection of extrapelvic lymph node metastases is high reported upto (100%); in contrast, sensitivity and specificity for lung and bone lesions were 75.0% (12/16) and 33.3% (1/3), respectively [75]. Chang et al. [71] reported detection of disease in 94% of patients with negative conventional imaging, leading to institution of earlier therapy.
Out of 150 patients who had CT or MRI scan findings suspicious for recurrent disease or elevated serum SCC-Ag levels, additional benefit from [18F]FDG PET was seen in 73.8%; in particular, [18F]FDG PET/CT corrected false negatives on CT-MRI in 74.2% and corrected false positives on CT-MRI in 25.8% of the cases. PET/CT was most useful in patients with lesions outside the pelvis [76].
Recent studies also support the usefulness of PET and its prognostic value in recurrent disease [77, 78]. In a retrospective analysis Definitive treatment rather than observation was undertaken in 37.5% of the patients (15/40). The most significant prognostic factor for death from cervical cancer was presence of more than three [18F]FDG-positive lesions, as the median survival for those with more than three PET-positive lesions was shorter as compared to those with a negative scan or one lesion on PET [77].
[18F]FDG PET increases physician confidence and decision making in those with equivocal findings on conventional imaging. [18F]FDG PET had a sensitivity of 92% and a specificity of 93% in those with other equivocal scans and increased experts’ confidence and diagnostic agreement from 68 to 98% [78].
[18F]FDG PET has a role in radiation treatment planning for patients with recurrent cervical cancers. By using PET/CT to identify metabolically active disease, the fields can be adjusted to reduce radiation exposure to the stomach, liver, and colon [79, 80] while maximizing radiation dose to the selective target, thereby better outcomes [63, 64]. Follow-up imaging with PET after treatment with radiation can help detect metabolically active disease [80].
[18F]FDG PET in Uterine Corpus Cancer/Carcinoma of Endometrium
Endometrial cancer is the most common female pelvic malignancy and the fourth most common cancer in women after breast, GI, and lung cancer; it is more common in postmenopausal women and in Western countries and is linked to exposure to unopposed estrogen. It commonly results in abnormal bleeding, which may be seen in about 80% of patients; about 15% of postmenopausal women presenting with abnormal bleeding have endometrial carcinoma [3, 4]. The uterine cancer most commonly presents as polypoid fungating masses in the endometrial cavity causing asymmetric enlargement of the uterus.
Invasion occurs into the myometrium, while distant spread of the tumor occurs through blood vessels or lymphatics. Prognostic factors include histology; histologic grade; depth of endometrial invasion; extension into cervix, vascular space, and adnexa; and pelvic node and aortic node involvement. Surgical staging is definitive and nodal sampling is routinely performed. Clinical staging is commonly based on FIGO classification (Table 22.3).
Table 22.3
Endometrial carcinoma: histopathologic types
1. Endometrioid adenocarcinoma (a) Papillary villoglandular (b) Adenocarcinoma with squamous differentiation (c) Secretory (d) Ciliated cell |
2. Mucinous adenocarcinoma |
3. Serous carcinoma |
4. Clear cell carcinoma |
5. Squamous carcinoma |
6. Undifferentiated carcinoma |
7. Mixed cell type |
8. Secondary tumors |
Common histology classifies the lesions into 8 categories (Table 22.4). Adenocarcinoma is the most common uterine cancer, seen as areas of irregular glands lined by malignant columnar epithelial cells. Although most adenocarcinomas are well differentiated, areas of squamous differentiation can occur and are prominent in adenoacanthomas. Squamous areas that are poorly differentiated and show cytologic features of malignancy are seen in adenosquamous carcinoma. Endometrial carcinomas are graded by their degree of histologic differentiation. Well-differentiated carcinomas are grade 1, while the presence of large solid areas qualifies the tumors as grade 2, and poor differentiation is grade 3. Both grades 2 and 3 carry a worse prognosis. Papillary serous adenocarcinoma is a variant that resembles ovarian serous carcinoma and has a worse prognosis than adenocarcinoma.
Table 22.4
AJCC and FIGO staging for endometrial cancer
Stage I: cancer is carcinoma confined to the corpus uteri |
IA: tumor limited to endometrium |
IB: invasion to less than 50% of the myometrium |
IC: invasion to >50% of the myometrium |
Stage II: cancer involves the corpus and the cervix but has not extended outside the uterus |
IIA: endocervical glandular involvement only |
IIB: cervical stromal invasion |
Stage III: extension beyond the uterus but not beyond the true pelvis |
IIIA: tumor invades serosa and/or adnexa and/or positive peritoneal cytology |
IIIC: metastases to pelvic and/or para-aortic lymph nodes |
Stage IV: involves the bladder or bowel mucosa or has metastasized to distant sites |
IVA: tumor invasion of bladder and/or bowel mucosa |
IVB: distant metastases, including intra-abdominal and/or inguinal lymph nodes |
Grading: |
• G1: no more than 5% of a nonsquamous or nonmorular solid growth pattern • G2: 6–50% of a nonsquamous or nonmorular solid growth pattern • G3: >50% of a nonsquamous or nonmorular solid growth pattern |
FIGO staging |
• Tumor limited to corpus |
– Stage IA; G123: tumor limited to endometrium – Stage IB; G123: invasion to less than 50% of the myometrium – Stage IC; G123: invasion to >50% of the myometrium |
• Tumor extends beyond the uterus, within the pelvis |
– Stage IIA; G123: endocervical glandular involvement only – Stage IIB; G123: cervical stromal invasion |
• Extension beyond the uterus but not beyond the true pelvis |
– Stage IIIA; G123: tumor invades serosa and/or adnexa and/or positive peritoneal cytology – Stage IIIB; G123: vaginal metastases – Stage IIIC; G123: metastases of pelvic and/or para-aortic lymph nodes |
• Extension to bladder and intestinal mucosa or distant metastasis |
– Stage IVA; G123: tumor invasion of bladder and/or bowel mucosa – Stage IVB; distant metastases including intra-abdominal and/or inguinal lymph nodes |
Leiomyosarcoma is a rare uterine neoplasm, accounting for 3% of all uterine malignant neoplasms. It is the most common uterine sarcoma, arising from smooth muscle of the myometrium. Leiomyosarcomas are generally bulky, fleshy masses that commonly occur in older women. These tumors have a high incidence of local recurrence and hematogenous metastases. Hemorrhage and necrosis are seen, with marked cytologic pleomorphism and atypia with a high rate of mitotic cells, which is diagnostic.
Role of [18F]FDG PET in Endometrial Carcinoma
[18F]FDG is known to accumulate in uterine tumors [81, 82]. Presurgical assessment with [18F]FDG PET for staging in high-grade endometrial cancer allows the detection of extrapelvic sites of disease and of metastasis to extra-abdominal organs. Preoperative [18F]FDG PET/CT detects primary lesions and distant disease with high sensitivity but has a lower sensitivity for the detection of nodal disease (57.1%) [83]. The specificity is high for both nodal disease (100%) and distant disease (96%). In endometrial cancer, SUVmax correlates with histological grade, tumor size, and Glut-1 expression. In 44 patients, the mean SUVmax of the primary endometrial cancer tumors was 17.6 (range, 3.04–34.74) [84]. In patients with high clinical risk and early-stage endometrial cancer, [18F]FDG PET has a high NPV. This may be useful in selecting patients who only may benefit from lymphadenectomy, thus minimizing operative and surgical complications. In 37 patients, pelvic node metastases were found at histopathological analysis in 9 (24.3%); patient-based sensitivity and specificity of [18F]FDG PET for detection of nodal disease were 77.8 and 100.0%, respectively, while nodal lesion site-based sensitivity and specificity were 66.7 and 99.4%, respectively [85]. Preoperative [18F]FDG PET with CTce is superior to conventional imaging in detecting metastatic disease but limited in assessment of lymph nodes, especially of small size. In 45 patients with endometrial carcinoma, the overall node-based sensitivity and specificity were 51.1 and 99.8%, respectively; for metastatic lymph nodes below 4 mm, the sensitivity was only 12.5%, while that for between 5- and 9-mm size was 66.7% (16/24), and that for 10 mm or larger was 100.0% (5/5) [86]. In uterine cancers [18F]FDG PET is useful for postsurgical monitoring and surveillance for recurrent disease (Fig. 22.4). Belhocine and colleagues analyzed findings in 34 women (41 scans) with suspected recurrence based upon tumor markers or findings of other imaging investigations. PET confirmed suspected recurrence in 88% of the cases; it also helped to localize the site of disease and detected asymptomatic recurrences in 12% of the patients. Moreover, in 9/26 of patients (35%), additional metastatic sites were detected with PET, a finding which significantly altered the treatment choice [87]. Therefore, [18F]FDG PET may be better than conventional imaging or tumor markers for recurrent disease. PET showed 100% sensitivity and 88.2% specificity, compared to 84.6% sensitivity and 85.7% specificity of conventional imaging or 100% sensitivity and 70.6% specificity of tumor markers. [18F]FDG PET affected the patient management in one-third of the cases, and patients with negative PET results had a trend to show longer disease-free courses [88]. A recent study compared CTld versus CTce-integrated PET imaging in recurrent uterine cancer and showed superiority of CTce in decreasing equivocal results, though no significant difference was noted in overall accuracy [68].
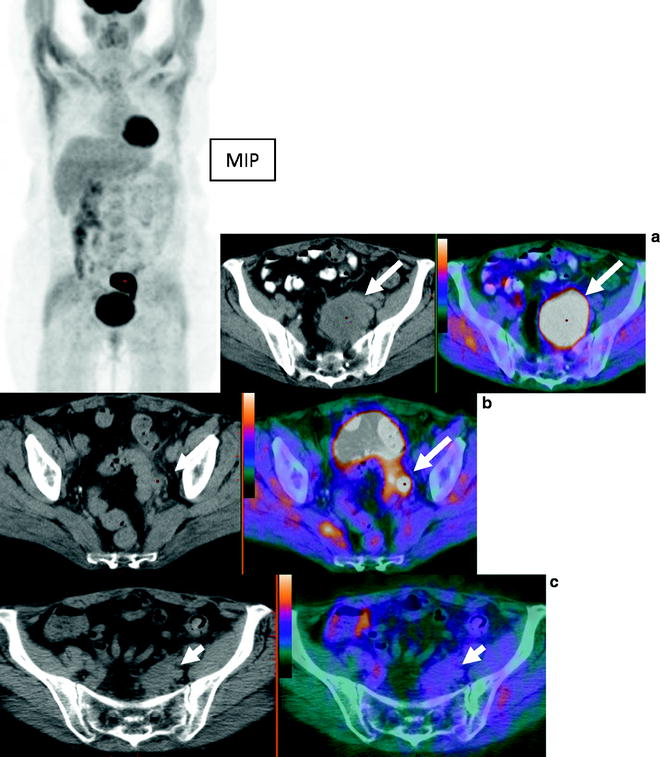

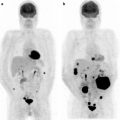
Fig. 22.4
A 57 year old woman with endometrial carcinoma was referred for evaluation of recurrent disease. MIP images show large area of abnormal activity superior to the bladder. Axial CT and fused PET/CT images show large left pelvic mass consistent with recurrent disease (a) and a node (b). Follow up study shows resolution of [18F]FDG avidity and decreased size of pelvic mass (c) post chemoradiation and subsequent surgical excision showed no viable tumor
Uterine sarcomas constitute less than 5% of the uterine corpus malignancies and, based on the type of cell they developed from, belong to three groups: endometrial stromal sarcomas, uterine leiomyosarcomas, and uterine carcinosarcomas. These tumors are very aggressive and have high mortality. MRI is presently the most accurate imaging modality. Umesaki and colleagues showed [18F]FDG uptake in leiomyosarcoma [89]. When compared with MRI in 5 patients, PET was positive in all 5 patients versus 4 for MRI. The uptake in most cases was high, with a mean SUV of 4.5 ± 1.3 [89]. [18F]FDG PET was performed preoperatively in 53 patients including sarcomas, endometrial carcinomas, and leiomyomas. [18F]FDG uptake measured as SUVs for uterine sarcomas and endometrial carcinomas is higher than those for leiomyomas; however, there was no significant difference in SUVs among endometrial carcinomas, carcinosarcomas, and leiomyosarcomas [90]. The diagnostic accuracy for leiomyosarcomas was only 73%. Sarcomas had higher Glut-1 expression than endometrial carcinomas. Data defining the role of [18F]FDG PET in routine clinical use is emerging and may play a useful role in staging and detection of recurrent disease.
Ovarian Cancer
The American Cancer Society estimated 21,550 new cases of ovarian cancer and 14,600 deaths related to ovarian cancer in 2009 in the United States. Ovarian cancer is the ninth most common cancer in women (not counting skin cancer) and ranks fifth as the cause of cancer death in women. Ovarian cancer is an insidious disease, and patients often present with an advanced stage of disease. Despite clinical advances and improved surgical techniques, it remains the form of gynecologic malignancy that has the highest mortality (Fig. 22.5). Ovarian cancer represents the second most common gynecologic malignancy, with a prevalence of 30 to 50 per 100,000 women, and leads to more than half of all deaths related to gynecologic cancers. There is no reliable screening tumor marker, and most cases present as advanced disease. Surgical debulking is critical, and complete cytoreduction is associated with longer disease-free survival than suboptimal debulking. In both cases, surgery is followed by chemotherapy. However, there is a high recurrence rate, and overall 5-year survival for advanced disease is only 17% [3, 4].
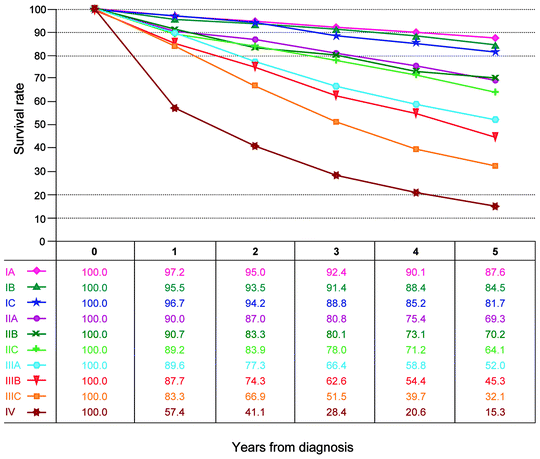

Fig. 22.5
Observed survival rates for 11,738 cases with primary ovarian epithelial cancer. Data from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) diagnosed in years from 1998–2002. Stage 0 includes 60 patients; stage IA, 1,415; stage IB, 160; stage IC, 878; stage IIA, 211; stage IIB, 304; stage IIC, 473; stage IIIA, 284; stage IIIB, 425; stage IIIC, 3,815; and stage IV, 3,773. (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC. http://www.springer.com)
Pathogenesis
The pathogenesis of ovarian cancer is multifactorial [1–4]. Five to 10% of ovarian cancers are familial, and three distinct hereditary patterns have been identified: breast-ovarian cancers (genetically linked to mutations in the BRCA1 and BRCA2 tumor suppressor genes), site-specific ovarian cancer, and ovarian and nonpolyposis colon cancers, or Lynch syndrome II. The remaining 90% of ovarian cancers are sporadic.
Pathology
Ovarian neoplasms are classified according to their histogenesis (Table 22.5). Tumor derived from surface epithelium is most common, with almost 90% of ovarian cancers originating from germinal epithelium. Other tumors include germ cell neoplasms, stromal neoplasms, and metastases (forming the remainder 10% of ovarian cancers). Epithelial ovarian cancer subtypes include most commonly the serous, endometrioid, mucinous, clear cell, undifferentiated, and Brenner types. Serous subtype is the most common, accounting for approximately 40% of primary ovarian neoplasms. They occur in the age group from 15 to 50 years. Benign neoplasms tend to occur in younger women than malignant ones. Serous tumors are frequently bilateral; 25% of benign, 30% of low malignant potential (borderline), and 70% of malignant serous tumors are bilateral. Mucinous tumors account for 20% of ovarian neoplasms and occur commonly in the age group from 15 to 50 years; these are mostly benign and more commonly unilateral. Mucinous cystadenocarcinoma accounts for 10% of ovarian cancers.
Table 22.5
Histologic classification of ovarian cancer
1. Epithelial tumors (a) Serous tumors • Adenocarcinoma • Surface papillary adenocarcinoma • Malignant adenofibroma and cystadenofibroma (b) Mucinous tumors • Adenocarcinoma • Malignant adenofibroma (c) Endometrioid tumors • Adenocarcinoma • Adenoacanthoma • Adenosquamous carcinoma • Adenosarcoma • Stromal sarcoma • Carcinosarcoma • Undifferentiated sarcoma (d) Clear cell tumors (e) Transitional tumors (f) Squamous cell tumors (g) Mixed epithelial tumors (h) Undifferentiated carcinoma |
2. Germ cell tumors (a) Teratoma (b) Dysgerminoma (c) Yolk sac carcinoma (d) Embryonal carcinoma (e) Choriocarcinoma |
3. Gonadal stromal tumors (a) Granulosa theca cell (b) Fibrothecoma (c) Sertoli-Leydig cell |
4. Mixed germ and Sertoli cell tumors |
5. Metastatic tumors |
Endometrioid carcinoma (which histologically resembles endometrial carcinoma) accounts for 20% of malignant ovarian neoplasms and is frequently bilateral; associated endometriosis is found in about 25% of the cases. In some cases concurrent endometrial carcinoma is present, thus raising the question of whether the ovarian neoplasm is metastatic or a second independent primary tumor. Origin of some endometrioid carcinomas from endometriosis has been demonstrated; believed to represent endometrioid differentiation of a neoplasm derived from the celomic epithelium. Endometrioid carcinomas grossly appear as solid and cystic masses that frequently show areas of hemorrhage and necrosis. Squamous metaplasia is seen in 50% of the cases [3, 4].
Staging
The staging system remains surgically based. Both the International Federation of Gynecology and Obstetrics (FIGO) and the TNM classification of ovarian cancer are based upon the intraoperative findings [5] (Table 22.6). Early-stage ovarian cancer is treated with comprehensive staging laparotomy, whereas advanced but operable disease is treated with primary cytoreductive surgery (debulking) followed by adjuvant therapy. Patients with unresectable disease may benefit from neoadjuvant (preoperative) chemotherapy before debulking. Factors that generally indicate inoperable disease include invasion of pelvic sidewall, rectum, sigmoid colon, or bladder; tumor deposits >1–2 cm in the porta hepatis, intersegmental fissure of the liver, lesser sac, gastrosplenic ligament, gastrohepatic ligament, subphrenic space, root of mesentery, and presacral space; suprarenal adenopathy; and hepatic (parenchymal), pleural, or pulmonary metastases.
Table 22.6
TNM and FIGO staging classification of ovarian cancer
Primary tumor (T) | |||
|---|---|---|---|
TNM categories | FIGO stages | Description | |
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
T1 | I | Tumor limited to ovaries (one or both) | |
T1a | IA | Tumor limited to one ovary; capsule intact, no tumor on ovarian surface. No malignant cells in ascites or peritoneal washing | |
T1b | IB | Tumor limited to both ovaries; capsules intact, no tumor on ovarian surface. No malignant cells in ascites or peritoneal washings | |
T1c | IC | Tumor limited to one or both ovaries with any of the following: capsule ruptured, tumor on ovarian surface, malignant cells in ascites or peritoneal washings | |
T2 | II | Tumor involves one or both ovaries with pelvic extension and/or implants | |
T2a | IIA | Extension and/or implants on uterus and/or tube(s). No malignant cells in ascites or peritoneal washings | |
T2b | IIB | Extension to and/or implants on other pelvic tissues. No malignant cells in ascites or peritoneal washings | |
T2c | IIC | Pelvic extension and/or implants (T2a or T2b) with malignant cells in ascites or peritoneal washings | |
T3 | III | Tumor involves one or both ovaries with microscopically confirmed peritoneal metastasis outside the pelvis | |
T3a | IIIA | Microscopic peritoneal metastasis beyond pelvis (no macroscopic tumor) | |
T3b | IIIB | Macroscopic peritoneal metastasis beyond pelvis <2 cm in greatest dimension | |
T3c | IIIC | Peritoneal metastasis beyond pelvis >2 cm in greatest dimension and/or regional lymph node metastasis | |
Regional lymph nodes (N) | |||
TNM categories | FIGO stages | ||
NX | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | IIIC | Regional lymph node metastasis | |
Distant metastasis (M) | |||
TNM categories | FIGO stages | ||
MX | Distant metastasis cannot be assessed | ||
M0 | No distant metastasis | ||
M1
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |||