Head Trauma
Jason F. Talbott
Alisa Gean
Traumatic brain injury (TBI) is a major cause of morbidity and mortality worldwide. In the United States, the Centers for Disease Control and Prevention estimates that 1.7 million people suffer TBI annually with combined direct and 13.12 indirect medical costs totaling >$60 billion annually (1). By the year 2020, injuries including TBI are estimated to account for 20% of worldwide burden of death and disability. As a result, considerable effort has focused on improving the prevention, accurate diagnosis, prognosis, and management of TBI patients. Effective diagnosis, treatment, rehabilitation, and prognostication for TBI are dependent upon the accurate characterization of initial injury severity. To complement standard clinical outcome measures, neuroimaging has played an increasingly integral role in injury characterization.
Clinical history, exam findings, and demographic data remain the cornerstone of initial TBI patient assessment. Since 1974, the most widely used clinical indicator of head injury has been the Glasgow Coma Scale (GCS) score. The GCS score may range from 3 to 15 based upon three components of neurologic function: (1) eye opening to external stimuli, (2) motor response to stimuli, and (3) verbal response (Table 13.1). One of the important limitations of this scale is that different varieties of traumatic lesions can produce similarly low GCS scores at admission. For example, initial low GCS scores may be seen with subdural hematomas (SDHs), epidural hematomas (EDHs), cortical contusions, intracerebral hematomas (ICHs), and traumatic axonal injury (TAI). As a result, confident predictions of outcome are difficult to establish within the first 24 hours of admission using the GCS.
TABLE 13.1 Glasgow Coma Scale | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Valid prognostic models at the time of admission are essential for early clinical decision-making and for research purposes. More recently, a prediction model based on admission characteristics leveraging data from several large patient series (more than 8,500 patients) available as part of the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) project has been developed and externally validated (2). As part of this study, the authors found that head computed tomography (CT) characteristics were an important component of the core prognostic data for predicting patient outcomes at 6 months after injury. Contemporaneously, admission data for TBI patients from the Corticosteroid Randomization After Significant Head injury (CRASH) trial (including more than 6,600 patients) similarly identified the importance of admission CT findings for TBI prognostication (3). While the role of noncontrast head CT has been well-established in triage evaluation of TBI, value added by emerging magnetic resonance imaging (MRI) technologies are being increasingly realized (4). Rapid advancement in MRI speed, coupled with development of MRI sequences ever more sensitive to TBI pathology, is providing insight into brain injury previously unidentified, particularly in the setting of mild injury (4), which accounts for the vast majority of TBI cases.
In this chapter, we briefly review the epidemiology and pathophysiologic mechanisms of TBI. We will then focus on the role of MRI in evaluating patients with head injury, highlighting the application of both conventional and advanced MRI techniques. The capabilities and limitations of MRI for imaging these patients are addressed. The classification, mechanisms, and pertinent clinical features of different types of traumatic lesions are also reviewed. Important imaging signs that can assist in predicting outcome from head injury are emphasized.
Epidemiology of Head Injury
The most common cause of death and permanent disability in the first few decades of life is trauma, and the neurologic components of trauma are responsible for most of these deaths and disabilities. In the United States, the annual incidence of TBI has been estimated at 180 to 250 per 100,000 population. As the leading cause of disability in people under 40, there are 15 to 20 per 100,000 TBI-related disabilities every year. The incidence of head injury peaks at 550 per 100,000 population for ages 15
to 24 years. The incidence then declines slightly until the age of 50 years, when it again starts to increase. About 500.000 cases of head injury can be expected to occur in the United States each year. However, these estimates almost certainly underrepresent the true incidence of TBI when accounting for all cases of mild TBI, which accounts for the vast majority of head injuries (5). For example, with sports-related concussion, a lack of systematic reporting and tendency to underreport when there has been no loss of consciousness (LOC) leads to a discrepancy in estimates and actual incidence (4,6). The yearly incidence of sports-related TBI alone in the United States has ranged from 300,000 to 3.8 million (4).
to 24 years. The incidence then declines slightly until the age of 50 years, when it again starts to increase. About 500.000 cases of head injury can be expected to occur in the United States each year. However, these estimates almost certainly underrepresent the true incidence of TBI when accounting for all cases of mild TBI, which accounts for the vast majority of head injuries (5). For example, with sports-related concussion, a lack of systematic reporting and tendency to underreport when there has been no loss of consciousness (LOC) leads to a discrepancy in estimates and actual incidence (4,6). The yearly incidence of sports-related TBI alone in the United States has ranged from 300,000 to 3.8 million (4).
Within the severe spectrum of TBI, up to 10% of the new cases will be fatal. As many as 5% to 10% of those that survive the initial trauma will experience some degree of residual neurologic deficit (7). Head injury mortality rates are estimated at 25 per 100,000 population each year (7). Fatal head injuries are four times more common in males than females (7). Traffic-related injuries account for between 20% and 50% of head injury deaths (7). Gunshot wounds to the head are responsible for 20% to 40% of deaths. Falls and nonfirearm assaults account for most of the remaining deaths (7). Falls typically account for a higher percentage of injuries at both extremes of life. Seventy-five percent of head injuries in preschool children are secondary to falls (7). Falls are also responsible for the vast majority of head injuries in the elderly population. Two-thirds of all head injury deaths occur before hospitalization (7). Measures designed to reduce mortality from head injury, therefore, will be unproductive unless preventative actions are also incorporated (7).
Pathophysiology of TBI
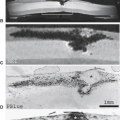
Neurotrauma is unique among brain disorders with respect to the critical role that biomechanics contributes to the understanding of injury type and severity (8). Holbourn’s early pioneering work concerning the mechanisms of head injury in adults has served as the fundamental basis for understanding the means by which traumatic stresses produce cerebral injury (Fig. 13.1) (9,10). Using a gelatin model of the brain, Holbourn concluded that injury to the brain occurred through two major mechanisms: direct injuries due to skull distortion (contact phenomenon) and indirect or inertial injuries that arise irrespective of skull deformation. The former is produced by localized fracture or inbending of the skull with direct injury to adjacent brain parenchyma. Neural damage of this type is typically superficial and localized to the immediate vicinity of the calvarial injury and almost always isolated to moderate and severe injuries. Examples of lesions caused by this mechanism are cortical lacerations and contusions due to depressed fracture fragments, and EDHs secondary to lacerations of meningeal arteries.
The second mechanism of injury relates to inertial forces which operate irrespective of skull deformation and may produce extensive neural damage, even in the absence of a direct blow to the head. Two related strain metrics applied to model brain deformation after traumatic injury include (1) pressure-induced volumetric strain and (2) shear strain. Largely composed of water, the brain tissue is highly resistant to pressure-induced volumetric deformation and thus volumetric strain does not significantly contribute to TBI pathology and will not be considered further (8). In contrast, shear-strain deformation is characterized by a change
in shape without a change in volume and is responsible for most mechanically induced TBI lesions. Because of their inherently low rigidity, neurons are extremely susceptible to shear-strain deformations. These shear strains develop because of differential movements of one portion of the brain with respect to another. Shear–strain forces are greatest at the junction of tissues that differ in density and rigidity (e.g., CSF–brain, gray–white matter, brain–pia-arachnoid, pia-arachnoid–dura, skull–dura).
in shape without a change in volume and is responsible for most mechanically induced TBI lesions. Because of their inherently low rigidity, neurons are extremely susceptible to shear-strain deformations. These shear strains develop because of differential movements of one portion of the brain with respect to another. Shear–strain forces are greatest at the junction of tissues that differ in density and rigidity (e.g., CSF–brain, gray–white matter, brain–pia-arachnoid, pia-arachnoid–dura, skull–dura).
Early investigations on shear strain focused on linear accelerations measured during impact in animal models of TBI and correlations with pressure recordings within brain surrogates (11). A strong correlation between peak pressure, peak acceleration, and neurologic dysfunction was established (12). Similar studies examining embalmed human cadavers identified injury thresholds for skull fracture and intracranial pressure thresholds, resulting in a human injury tolerance curve, commonly referred to as the Wayne State Tolerance Curve (WSTC). The WSTC, which is based on linear acceleration thresholds, ultimately formed the basis for testing standards for protective equipment design and automotive safety systems (8).
Rotational acceleration is the second form of inertial loading contributing to shear-strain injury experienced by the brain at the moment of impact. Animal studies, largely performed subsequent to the development of the WSTC, have validated the early pioneering work of Holbourn and led to the now commonly accepted wisdom that shear strain secondary to rotational acceleration is largely responsible for the majority of traumatic cerebral injury (13). In these studies, primates subjected to high-magnitude angular inertial forces developed widespread axonal injury and prolonged unconsciousness. The complex, interconnected, oblique, and orthogonal orientations of many organized fiber bundles in the brain make its structure highly susceptible to diffuse injury as a result of shear forces resulting from rotational acceleration. This phenomenon can explain the often diffuse distribution of TAI, even in the absence of direct contact injury. Although traumatic coma is very difficult to produce when the head is constrained to exclude rotational motion, allowing rotational acceleration markedly increases the risk of TAI and an unconscious episode (14). However, the precise relative contributions of linear and rotational accelerations as well as other impact factors to neurologic dysfunction remain under active debate.
Unlike traumatic lesions from contact phenomenon, rotationally induced shear-strain lesions are typically multiple, bilateral, and widespread. They can occur remote from the site of impact, and may be either superficially or deeply situated (Figs. 13.2–13.5) (9,10). The location of lesions produced by this mechanism, as would be expected, closely corresponds to the locations of maximum shear strains that develop during rotational acceleration of the head. The expected anatomic distribution was experimentally mapped by Holbourn (9,10) for the three orthogonal planes of rotation using a gelatin brain model (Fig. 13.1). Despite the limitations of this model, the distribution of traumatic lesions in fatal head injuries and animal trauma models has generally followed Holbourn’s theoretical predictions. Figure 13.1 illustrates the location of maximum shear strain for all three orthogonal planes of rotation (9,10). Rotationally induced shear-strain injury typically produces lesions at one of the four topographic levels: cortical surface of brain (contusions), TAI, brainstem, and penetrating blood vessels (arteries or veins). The predominate types of lesions observed in a particular patient are determined by the specific mechanical circumstances present during trauma. Although neurons are the most susceptible tissue to rotationally induced shear-strain deformations, non-neuronal tissues (glia, penetrating blood vessels, bridging veins, pia-arachnoid) may also be injured by this mechanism (9,10).
In addition to biomechanical mechanisms, an understanding of the molecular and cellular changes underlying TBI can
inform our radiologic evaluation of underlying pathology. The biomechanical forces outlined above produce a primary injury which directly disrupts the normal structure and function of neurons, glia, and blood vessels. Subsequent to this primary insult, a vast array of secondary injury processes are initiated resulting in complex molecular and cellular alterations which may persist for days, weeks, and months after injury (15). Mechanical disruption of both neurons and glial cells results in distortions of membrane-bound ion channels with loss of normal electrochemical gradients necessary for maintenance of cell function. Associated mitochondrial failure depletes ATP supply and prohibits energy-dependent restoration of ionic gradients. Simultaneously, disruption of the vasculature results in opening of the blood–brain barrier and vasogenic edema. Loss of vascular autoregulatory mechanisms contributes to hypoxic conditions with resultant ischemia and cytotoxic injury (7,15,16,17,18). Widespread neuronal depolarizations are also implicated in expression of immediate early genes including those for excitotoxic neurotransmission and neuroinflammatory genes which further exacerbate tissue damage (19). At the axonal level, varying degrees of shear strain influence the extent of TAI (15). With less than 5% strain, transient axonal depolarizations occur with full membrane and functional recovery. Greater than 20% strain leads to membrane fragmentation, cytoskeletal breakdown, and primary axotomy. Intermediate strain levels (5% to 20%) activate a complex cascade of ionic fluxes, cytoskeletal disruptions, and myelin breakdown precipitated by axolemmal damage (15). These processes can occur over hours to weeks. As Bigler and Maxwell point out in their recent review, a broad appreciation of TBI pathophysiology is important for interpretation of neuroimaging studies (15). For example, alterations in blood-oxygen level–dependent (BOLD) contrast are noted after TBI in a growing body of functional MRI (fMRI) literature. Such findings may reflect, in part, primary neural damage, but consideration of microvascular pathology and autoregulatory dysfunction must be integrated into the interpretation of these results (15). We are entering an exciting era in neuroimaging where advanced imaging tools are beginning to noninvasively tease out these molecular and cellular underpinnings of TBI.
inform our radiologic evaluation of underlying pathology. The biomechanical forces outlined above produce a primary injury which directly disrupts the normal structure and function of neurons, glia, and blood vessels. Subsequent to this primary insult, a vast array of secondary injury processes are initiated resulting in complex molecular and cellular alterations which may persist for days, weeks, and months after injury (15). Mechanical disruption of both neurons and glial cells results in distortions of membrane-bound ion channels with loss of normal electrochemical gradients necessary for maintenance of cell function. Associated mitochondrial failure depletes ATP supply and prohibits energy-dependent restoration of ionic gradients. Simultaneously, disruption of the vasculature results in opening of the blood–brain barrier and vasogenic edema. Loss of vascular autoregulatory mechanisms contributes to hypoxic conditions with resultant ischemia and cytotoxic injury (7,15,16,17,18). Widespread neuronal depolarizations are also implicated in expression of immediate early genes including those for excitotoxic neurotransmission and neuroinflammatory genes which further exacerbate tissue damage (19). At the axonal level, varying degrees of shear strain influence the extent of TAI (15). With less than 5% strain, transient axonal depolarizations occur with full membrane and functional recovery. Greater than 20% strain leads to membrane fragmentation, cytoskeletal breakdown, and primary axotomy. Intermediate strain levels (5% to 20%) activate a complex cascade of ionic fluxes, cytoskeletal disruptions, and myelin breakdown precipitated by axolemmal damage (15). These processes can occur over hours to weeks. As Bigler and Maxwell point out in their recent review, a broad appreciation of TBI pathophysiology is important for interpretation of neuroimaging studies (15). For example, alterations in blood-oxygen level–dependent (BOLD) contrast are noted after TBI in a growing body of functional MRI (fMRI) literature. Such findings may reflect, in part, primary neural damage, but consideration of microvascular pathology and autoregulatory dysfunction must be integrated into the interpretation of these results (15). We are entering an exciting era in neuroimaging where advanced imaging tools are beginning to noninvasively tease out these molecular and cellular underpinnings of TBI.
Relative Roles of Imaging Studies for Head Trauma Imaging
In the setting of acute head trauma, CT remains the imaging standard of care for evaluation of lesions requiring immediate neurosurgical intervention (i.e., acute EDH) (20). Advantages of noncontrast CT imaging for acute head trauma include rapid acquisition, widespread availability, and lack of any significant contraindications. As a diagnostic exam, CT provides excellent sensitivity for demonstrating mass effect, acute hemorrhage, bone injury, and ventricular size/displacement (21,22). For these reasons, CT remains the diagnostic study of choice for the initial evaluation of TBI patients (Table 13.2) (20). This is likely to be true for the immediate future, especially for the patient with
multiple-organ trauma. While CT imaging for acute moderate and severe TBI is clearly indicated, guidelines for screening CT in mild TBI (mTBI) are less well defined. Indications for head CT in patients with mTBI (defined as GCS 13 to 15) according to most major guidelines include LOC >30 seconds to 1 minute, prolonged altered or deteriorating level of consciousness, severe headache, focal neurologic deficit or seizure, or worsening symptoms (4).
multiple-organ trauma. While CT imaging for acute moderate and severe TBI is clearly indicated, guidelines for screening CT in mild TBI (mTBI) are less well defined. Indications for head CT in patients with mTBI (defined as GCS 13 to 15) according to most major guidelines include LOC >30 seconds to 1 minute, prolonged altered or deteriorating level of consciousness, severe headache, focal neurologic deficit or seizure, or worsening symptoms (4).
TABLE 13.2 Diagnostic Flow Chart for Evaluation of Acute Head Injury Patients | |
|---|---|
|
The role of MRI in head trauma evaluation is evolving. Factors that limit the widespread use of MR as the primary diagnostic study for evaluation of trauma patients include its limited availability in the acute trauma setting, long imaging times relative to CT, sensitivity to patient motion, greater cost, greater difficulty of patient monitoring, lower sensitivity for detecting fractures, and physician unfamiliarity with the MR appearance of traumatic lesions (23,24,25,26). An additional major drawback to the use of MR in head trauma has been the logistic difficulty of safely imaging severely injured patients in the MR environment (27). These limitations have been greatly reduced, or eliminated, during the last few years and now offer little impediment to the use of MR for evaluating acute TBI patients (16,17). Notable advances include the development of self-shielded magnets, wider and more accessible scanning gantries, and a wide range of nonferromagnetic life-support and monitoring devices that are compatible with the MR environment. These developments have greatly facilitated the evaluation of these critically ill patients. Every physiologic parameter that might need to be monitored in these patients can be safely monitored (7). Sensors for monitoring respiratory rate and effort, invasive or noninvasive blood pressure, arterial oxygen saturation (pulse oximetry), heart rate and rhythm, end-expiratory CO2 levels, electrocardiographic activity, temperature, and intracranial pressure are available. Multiple types of respirators, ventilators, and full anesthesia machines that are MR compatible are also available. The radiologist should be familiar with the many important concerns, principles, and techniques regarding patient monitoring in the MR environment.
With these considerations in mind, the decision to use MRI for diagnostic evaluation of TBI patients largely relies on the clinical scenario. While the most appropriate imaging test must be tailored to each individual patient, several general guidelines can be provided (Table 13.2). A significant factor to consider is the severity of injury as assessed by the GCS. As mentioned above, neurotrauma patients are routinely categorized into three clinical subgroups, depending on the degree of impairment of the admission GCS score: mild (GCS = 13 to 15), moderate (GCS = 9 to 12), and severe (GCS <9) (22).
Mild acute TBI (mTBI) is by far the largest subgroup of TBI patients, representing at least 80% of all traumatic head injuries, and likely much greater given the frequency of underreporting (6). Although the clinical definition for mTBI is variable, patients generally present after head trauma with transient altered consciousness of less than 30-minutes duration, posttraumatic amnesia not exceeding 24 hours, and GCS of 13 to 15 at presentation. The term “concussion” is often used in the sports literature synonymously with mTBI, although the absence of intracranial structural abnormalities on CT and conventional MRI sequences is implied. When traumatic intracranial lesions are identified on CT in patients with GCS 13 to 15, the term complex mTBI is often applied. Some clinicians maintain that mild head injury patients can be safely managed by clinical observation alone without initially obtaining any imaging study (22). Others, however, strongly advocate early diagnostic evaluation. As described above, according to most major clinical guidelines, indication for acute head CT include LOC for >30 seconds to 1 minute, prolonged altered consciousness, severe headache, seizure or other focal neurologic deficit, or worsening symptoms (4) (Table 13.2). This subgroup of patients has a 5% to 30% incidence of intracranial hemorrhage on initial head CT (22). Mild TBI patients are less likely than those with more severe degrees of injury, however, to have significant intraparenchymal lesions. For patients meeting clinical criteria for mTBI, CT is the most efficacious method for evaluating the lesions for many reasons including availability, speed, and high sensitivity and specificity for both fracture and intracranial hemorrhage (7). Conventional MRI sequences have been shown in numerous studies to have superior sensitivity compared with CT for detecting many types of traumatic injury in these patients, including TAI, small contusions, and small extra-axial hematomas (7,28). As a result, MRI is appropriate in the acute setting of mTBI when the clinical symptomology does not match the CT imaging findings (Table 13.2) (20). Moreover, due to its superiority for detection and characterization of nonsurgical injuries, MRI is recommended for evaluation of mTBI patients with persistent symptoms in the subacute and/or chronic stages of injury (20) (Table 13.2). Whereas these findings may not be known to alter the clinical management or outcome of mild head injury patients (22), there is increasing evidence that valuable prognostic information may be derived from subacute MRI (28,29,30). Although advanced MRI neuroimaging techniques, including diffusion tensor imaging (DTI), fMRI, susceptibility-weighted imaging (SWI), MR spectroscopic imaging (MRSI), and perfusion-weighted MRI (PW-MRI) may have utility in identifying and characterizing otherwise occult injury of brain trauma (discussed later in this chapter), these techniques are not yet recommended for guiding routine clinical management of mTBI patients (20).
The moderate and severe head injury categories share many clinical features and management concerns (7). These two groups, therefore, will be considered together. Neurologically unstable patients in these two categories should be initially studied with CT (7,20). These patients, by definition, already have significant impairment of consciousness and focal neurologic deficits that are deteriorating. The most critical issue in this situation is to rapidly detect potentially treatable hematomas or other surgically correctable lesions. Although CT may miss several important types of injuries, it is still the most efficient means of rapidly excluding surgical lesions (7). Moderate and severe head injury patients who are initially stable can be primarily evaluated by MR (7,16,17,27). It is advantageous to use MR in these patients, if possible, because it is considerably more sensitive for detecting most traumatic parenchymal and extraparenchymal lesions (7,17,27).
It is reasonable to suggest that all moderate to severe head injury patients should be evaluated with MR at some point in time during the first 2 weeks after injury (Table 13.2). The full extent of TBI will not be fully determined if only CT is used to evaluate this group of patient as MR is more valuable than CT for assessing the full magnitude of injury (7,17). It also provides more accurate information regarding the expected degree of final neurologic recovery (28,29). The MR exam can be done as the initial examination in stable patients (7,17,18,27). In unstable patients, however, it is probably the best to initially study the patients with CT and postpone the MR examination until they can be safely imaged. It is advisable to obtain the MR study within the first 2 weeks after injury, if possible, because most parenchymal lesions are more visible during this time period. Edema and axoplasmic leakage around areas of neuronal disruption are maximal during the first 2 weeks, rendering lesions more conspicuous. Smaller lesions are more difficult to detect over the ensuing weeks as intra- and extracellular edema gradually subsides (7). After the edema resolves, many traumatic intra-axial lesions may be quite indistinct on CT and standard T2-weighted MR scans (7).
CT remains the study of choice for the acute evaluation of neurologically or hemodynamically unstable patients who have significant impairment of consciousness (Table 13.2) (7).
The most critical issue in this situation is rapid detection of potentially treatable hematomas or other surgically correctable lesions. In unstable patients, it is unwise to spend even a few extra minutes to obtain an MR exam when CT can more quickly and safely answer the urgent questions. Similarly, CT can be used for evaluation of patients with rapid changes of neurologic status at any point in time after acute head injury, although emergency MR is increasingly common in this setting when available (Table 13.2).
The most critical issue in this situation is rapid detection of potentially treatable hematomas or other surgically correctable lesions. In unstable patients, it is unwise to spend even a few extra minutes to obtain an MR exam when CT can more quickly and safely answer the urgent questions. Similarly, CT can be used for evaluation of patients with rapid changes of neurologic status at any point in time after acute head injury, although emergency MR is increasingly common in this setting when available (Table 13.2).
Magnetic Resonance Imaging Strategies and Techniques
Conventional Imaging Protocols
The optimal MR protocol for a specific acute head injury patient varies, depending on the individual circumstances. The patient’s clinical condition must always be of principal concern. It may be necessary to delay the MR exam or substitute a CT in some patients if they are neurologically unstable (Table 13.2). When the MR study is performed, it is best to obtain the MR images as rapidly as possible. A judgment must often be made as to how much time can be safely permitted for answering all critical questions. An abbreviated MR examination, tailored to address the most crucial questions in the shortest time possible, may be necessary in some cases.
With the aforementioned considerations in mind, the MR exam should be structured so that it can detect all intracranial hematomas, identify nonhemorrhagic forms of injury, provide sufficient anatomic information to classify lesions, guide surgical treatment, and provide an estimation of long-term prognosis (7,17,18,31). The primary emphasis should be directed at maximizing the sensitivity of the examination because these objectives can only be met if all traumatic lesions are accurately identified. Recently, experts in TBI convened as part of the “Advanced Integrated Research on Psychological Health and Traumatic Brain Injury: Common Data Elements (CDE)” joint workshop (31). One task of this expert panel was to define a standardized MRI protocol for studying TBI. MRI protocols including conventional (tier 1) and advanced (tiers 2 to 4) MRI sequences were developed for 1.5 and 3 Tesla (T) (31). Tier 1 protocols are designed for routine clinical applications whereas tiers 2 to 4 are designed for research applications (31). A recommended clinical protocol tailored for TBI evaluation at 1.5 T (tier 1) incorporates multiplanar T1-weighted (3D T1 if available), T2-weighted, T2 FLAIR–weighted, and T2*-weighted gradient-recalled echo (GRE) or SWI sequences, in addition to standard DWI. At 3 T, the use of 3D T2-weighted imaging is recommended in addition to the above for high-resolution multiplanar localization of traumatic lesions.
Multiple imaging planes are very beneficial for detection and characterization of traumatic lesions (7,31). For example, superficial lesions are most reliably detected when the imaging plane is perpendicular to the cortical base of the lesion (16). Multiplanar imaging is also helpful for the detection of small lesions (e.g., traumatic brainstem lesions) because they may be missed due to partial volume effects or an interslice gap (18). Multiple planes are also essential for determining the exact location of traumatic lesions. Precise localization is necessary for accurate classification of lesions, which in turn has great impact on defining the long-term prognosis. For example, it can be quite difficult with only one plane of imaging to determine whether a small lesion close to the cortical surface of the brain is a cortical contusion or a peripheral TAI lesion. Only when the imaging plane is perpendicular to the adjacent calvarium can this be reliably established. It is desirable to have both T1- and T2-weighted scans in at least two perpendicular planes for precisely localizing and classifying traumatic lesions, another advantage to 3D acquisitions.
The visibility of a lesion on a particular pulse sequence is influenced by a number of factors: lesion size and location, presence and age of hemorrhage, presence of edema, and MR acquisition parameters (16). The most important factor that affects lesion visibility is the MR pulse sequence used (16). T1-weighted imaging is commonly employed to map brain anatomy. Usually limited to research applications, 3D T1 sequences may also be utilized for quantitative volumetric analysis of the brain. Increased water content in the brain evidenced by T2 prolongation generally has an inverse effect on T1 signal with decrease in T1 intensity. T1 imaging can be very helpful for identification of subacute blood products secondary to the T1-shortening effect of methemoglobin. Contrast-enhanced T1-weighted imaging may identify areas of trauma-induced increase in blood–brain barrier permeability beginning 5- to 6 days after injury (Fig. 13.3). Moreover, pachymeningeal hyperenhancement following TBI has been described as a sensitive indicator of TBI. However, T1-weighted postcontrast imaging does not improve sensitivity for TBI lesions compared with conventional sequences and is not recommended as part of routine TBI MRI protocol (31). Nevertheless, postcontrast imaging may have value as an optional sequence for helping establish chronicity of injury and for following evolution of TBI lesions when other nontraumatic intracranial pathologies are under consideration.
In general, T2-weighted imaging is sensitive to pathology, but it can be nonspecific with respect to etiology. An increase in water content, as seen with vasogenic edema, manifests as T2 prolongation and increased T2 signal intensity. T2 imaging is also sensitive to the paramagnetic effects of iron in the form of hemosiderin. Phase dispersion results in T2 shortening and hypointense signal. Importantly, T2 imaging is superior in its sensitivity to TAI lesions compared with CT (31). Fast spin-echo (FSE) T2-weighted images, rather than conventional SE images, allow images with T2-weighting to be obtained in a fraction of the time required for conventional SE techniques and can be implemented on conventional scanners (Figs. 13.4 and 13.5) (7). Because FSE uses radiofrequency refocusing pulses to generate echoes, true T2 contrast is maintained. The FSE sequence, however, is less prone to magnetic susceptibility–induced artifacts than T2*-weighted GRE sequences that use gradient refocusing to generate echoes (7). Clearly, this is disadvantageous in the case of TBI, where it is desirable to identify hematomas by their distinctive areas of susceptibility-induced hypointensity arising from paramagnetic iron (Fig. 13.5). Therefore, it is recommended that all patients be imaged with GRE or SWI scanning as a supplement to an FSE T2-weighted sequence.
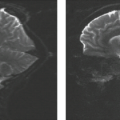
FLAIR T2-weighted imaging highlights both parenchymal lesions that abut the ventricles, subarachnoid space and extra-axial hemorrhage by virtue of suppression of normal cerebrospinal fluid (CSF) signal (Fig. 13.2). The intrinsic T2 weighting with FLAIR is highly sensitive for evaluation of edema, both vasogenic and cytotoxic in nature. FLAIR and standard T2-weighted imaging are also useful for identifying iron content in the form of hemosiderin, when hypointense signal is present. FLAIR and T2 images may also identify foci of TAI that are beyond sensitivity of CT (28,32). Recently, Kim and colleagues demonstrated high sensitivity for contrast-enhanced FLAIR imaging for identification of posttraumatic pachymeningeal hyperenhancement (33). This conspicuous finding alerts the physician to the detection of additional, more subtle, traumatic intracranial lesions, including trace subarachnoid and subdural hemorrhage.
DWI is sensitive to microscopic motion of water molecules in tissue and has demonstrated utility for identifying foci of TAI (34). In the acute setting, DWI has been shown to have superior sensitivity for detection of traumatic white matter injury compared with FLAIR and T2* GRE sequences
(Figs. 13.6–13.8). Moreover, apparent diffusion coefficient (ADC) values can readily distinguish cytotoxic from vasogenic edema in the acute and subacute phases and allows for highly sensitive detection of secondary acute ischemic infarction in the setting of TBI.
(Figs. 13.6–13.8). Moreover, apparent diffusion coefficient (ADC) values can readily distinguish cytotoxic from vasogenic edema in the acute and subacute phases and allows for highly sensitive detection of secondary acute ischemic infarction in the setting of TBI.
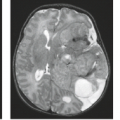
Accurate detection of hemorrhage also constitutes an important aspect of trauma imaging. MR was initially believed to be insensitive for detection of some hematomas. However, it is apparent that MR is extremely sensitive to hemorrhage throughout all stages of its evolution (16,35). The sensitivity of different MR pulse sequences to hemorrhage varies with the age of the hematoma and the specific biochemical nature of the hemoglobin (35), as well as with a variety of intrinsic and operator-dependent factors. Briefly, hyperacute hematomas less than 4 to 6 hours old will, for simplicity’s sake, be considered to be primarily composed of hemoglobin that is in a fully oxygenated state (oxyhemoglobin) (35). Oxyhemoglobin is diamagnetic and therefore (unlike deoxyhemoglobin, methemoglobin, and chronic iron storage forms such as hemosiderin) does not produce significant shortening of the T1, T2, or T2* relaxation time. Hyperacute hematomas, therefore, may have signal intensities that are quite similar to that of adjacent brain parenchyma or any nonparamagnetic lesion on all MR pulse sequences (35). It was initially believed that MR might not detect these relatively isointense lesions. It is now clear that few significant hyperacute hematomas will be overlooked if MR images are acquired with T1-, T2-, and T2*-weighted sequences (7,16). Invariably there is enough anatomic distortion, perifocal edema, and MR signal intensity difference between hyperacute hematomas and brain parenchyma to allow their recognition.
MR angiography (MRA) has become another important diagnostic tool for the evaluation of vascular pathologies of the central nervous system. In the last decade, there has been dramatic improvement of CT angiography (CTA) with multidetector scanners, and that has proceeded so rapidly that many trauma centers have shifted emergency evaluation of the neck and head vessels to CTA rather than MRA. The current role of MRA for evaluation of traumatic vascular injury, however, is noted later in this chapter.
 FIGURE 13.8 DWI lesion volume correlates with outcome. This 17-year-old male patient after TBI secondary to a motor vehicle crash. Total lesion extent, with abnormality involving the bilateral corona radiata, bilateral centrum semiovale, and corpus callosum, is better depicted on DWI (first row) than on T2-weighted images (second row) or GRE images (third row). Hemorrhagic foci (arrows) are best seen on the GRE images. This example demonstrates why lesion volume on DWI may have a higher correlation with clinical scores than does lesion number. This patient had fewer lesions than the patient in Figure 12.7, but he also had a much larger lesion volume on DWI and a higher score on the Modified Rankin Scale. (From Schaefer PW, Huisman TAGM, Sorensen AG, et al. Diffusion-weighted MR imaging in closed head injury: high correlation with initial Glasgow Coma Scale score and score on Modified Rankin Scale at discharge. Radiology 2004;233:58–66, with permission.) |
Advanced MRI Techniques
The last decade has witnessed rapid advancement in MRI sequence development and analysis techniques which are enabling the interrogation of microstructural, metabolic, and functional connections within the brain. While a complete review of the literature related to these advanced MRI techniques is beyond the scope of this chapter, notable advances in DTI, SWI, functional MRI (fMRI), magnetic resonance spectroscopy (MRS), and PW-MRI will be highlighted. It should be stated that presently, none of these advanced MR techniques are recommended by the American College of Radiology (ACR) appropriateness guidelines for routine evaluation of the TBI patient (20).
Diffusion Tensor Imaging (DTI)
With DTI, the 3D directionality of water motion can be modeled in tissues. The highly organized structure of white matter bundles in the brain strongly favors free diffusion of water along the orientation parallel to white matter bundles with relative reduction in diffusion perpendicular to axon bundles. Diffusion imaging with acquisition of at least six noncollinear diffusion gradient directions (usually many more) allows for tensor modeling with derivation of quantitative scalar metrics which reflect diffusion properties averaged over a voxel of tissue at the smallest scale (36). The most commonly utilized DTI parameters include fractional anisotropy (FA), a unitless scalar metric quantifying the degree of anisotropy within a voxel, and mean diffusivity (MD), the overall magnitude of diffusivity averaged over all sampled directions. Other parameters such as axial diffusivity (AD) (a measurement of diffusivity along the primary eigenvector of the tensor), and radial diffusivity (RD) (the averaged diffusivity along the two minor axes of the tensor ellipse) have been shown to correlate with microstructural white matter pathologies including axonal injury and demyelination, respectively (37,38). Analysis techniques for DTI datasets include histogram analysis, voxelwise analysis, region-of-interest analysis, and tractography. The reader is referred to excellent reviews on this topic for further detail (4). Extensive investigation has focused on utilizing DTI as a biomarker for investigating white matter microstructural alterations in TBI, specifically damage related to TAI (39).
A thorough review of the DTI literature related to TBI is beyond the scope of this chapter and the reader is referred to excellent reviews recently published on this topic (39,40,41). The heterogeneity with respect to patient populations, imaging parameters, analysis techniques, and imaging time points complicates the interpretation of TBI–DTI literature (39). For example, during the acute/semiacute stages of TBI, FA values within affected white matter regions have been shown to be normal (42), decreased (29), and increased (43) relative to normal controls. Such discrepancy may, to some degree, reflect underlying heterogeneity of the TBI syndrome, but also highlights the need for larger longitudinal studies incorporating standardized acquisition and analysis protocols and outcome measures (39). Despite these limitations, DTI has been validated as a robust measure of TAI at a group level, implicating TBI-related injury to frontal and temporal lobe association areas including the anterior corona radiata, uncinate fasciculus, superior longitudinal fasciculus, and anterior corpus callosum (Figs. 13.9 and 13.10) (40). Importantly, abnormal MD and FA measures in these regions have been shown to correlate with behavioral
and cognitive outcome measures at follow-up (29,44). Yuh and colleagues have recently demonstrated the prognostic utility of DTI in individual mTBI patients (Fig. 13.9). As part of a prospective multicenter study entitled Transforming Research And Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) pilot, the authors used both whole-brain voxelwise and selective region of interest (ROI) DTI analysis techniques in a heterogeneous mTBI patient population. DTI parameters surpassed CT, clinical, demographic, and socioeconomic variables as predictors of 3- and 6-month outcomes at both group and individual patient levels (29). Such studies will certainly soon lead to consensus guidelines for utilization of DTI parameters for diagnostic and prognostic purposes in individual TBI patients.
and cognitive outcome measures at follow-up (29,44). Yuh and colleagues have recently demonstrated the prognostic utility of DTI in individual mTBI patients (Fig. 13.9). As part of a prospective multicenter study entitled Transforming Research And Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) pilot, the authors used both whole-brain voxelwise and selective region of interest (ROI) DTI analysis techniques in a heterogeneous mTBI patient population. DTI parameters surpassed CT, clinical, demographic, and socioeconomic variables as predictors of 3- and 6-month outcomes at both group and individual patient levels (29). Such studies will certainly soon lead to consensus guidelines for utilization of DTI parameters for diagnostic and prognostic purposes in individual TBI patients.
Future directions in diffusion imaging research for TBI include improved standardization of DTI techniques and commencement of larger, longitudinal prospective studies utilizing standardized outcome measures. In addition, application of more advanced diffusion acquisitions and modeling algorithms, including multishell diffusion techniques like neurite orientation dispersion and density imaging (NODDI) and diffusion kurtosis imaging are under active investigation (45,46). Diffusion modeling algorithms utilizing these techniques are not constrained by the Gaussian tensor model and may add to the limited specificity currently provided with standard DTI metrics. Although in their infancy, such techniques may add additional information to conventional DTI parameters (47).
Susceptibility-Weighted Imaging (SWI)
SWI takes advantage of susceptibility differences between tissues to provide contrast. Filtered phase image data from a high-resolution, 3D velocity–compensated gradient-echo sequence
is combined with the magnitude image to produce the final SW image. The resulting SW image is exquisitely sensitive to blood products in hemorrhage, a property that has been exploited for evaluating TBI lesions (Fig. 13.11) (48,49). Compared with conventional GRE T2* sequences, SWI has been shown to be three to six times more sensitive for detection of hemorrhagic lesions (50). However, mixed results have been reported with respect to correlations between SWI findings and clinical outcomes. Compared with T2 and FLAIR images, Chastain and colleagues (51) showed that SWI is highly sensitive for hemorrhagic lesions, but does not discriminate between good and poor outcomes as measured by Glasgow Outcome Scores (GOS). In contrast, Spitz and colleagues more recently showed significantly increased sensitivity for hemorrhagic brain lesions compared with FLAIR and better correlation with some clinical measures of injury severity (52).
is combined with the magnitude image to produce the final SW image. The resulting SW image is exquisitely sensitive to blood products in hemorrhage, a property that has been exploited for evaluating TBI lesions (Fig. 13.11) (48,49). Compared with conventional GRE T2* sequences, SWI has been shown to be three to six times more sensitive for detection of hemorrhagic lesions (50). However, mixed results have been reported with respect to correlations between SWI findings and clinical outcomes. Compared with T2 and FLAIR images, Chastain and colleagues (51) showed that SWI is highly sensitive for hemorrhagic lesions, but does not discriminate between good and poor outcomes as measured by Glasgow Outcome Scores (GOS). In contrast, Spitz and colleagues more recently showed significantly increased sensitivity for hemorrhagic brain lesions compared with FLAIR and better correlation with some clinical measures of injury severity (52).
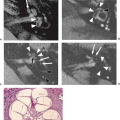
 FIGURE 13.10 DTI reveals white matter abnormalities in mTBI. FLAIR (A), fractional anisotropy (FA) map (B), and fiber tracking (C,D) in a 49-year-old patient imaged 16 months after the initial trauma. The FLAIR image shows no abnormalities. From the color-coded FA map (B), a region with reduced FA was identified in the white matter of the left frontal lobe. This region of interest (ROI), illustrated in the top right T2-weighted image, included forceps minor and frontotemporo-occipital fibers (C, superior oblique view). At the level of the ROI, the respective fibers are discontinuous (D, arrow). (From Rutgers DR, Toulgoat F, Cazejust J, et al. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. Am J Neuroradiol 2008;29:514–519, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|