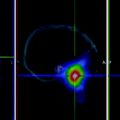
Fig. 1
Coronal fused 124I PET/CT images showing restoration of radioiodine uptake after Selumetinib therapy
Combining MEK inhibitor therapy with 124I dosimetry will help better select patients who will respond to 131I therapy and eliminate those who will not respond.
3 Fusion Imaging as a Basis for Theranostic Application to Radionuclide Therapy
Because PET is quantitative, positron-labeled forms of radio peptides are beginning to be widely used as part of a theranostic pair, of diagnostic and therapeutic application. In particular 68Ga-DOTATOC PET/CT has been used to predict potential utility in individual patients of radio peptide therapy with 177Lu-Octreotate (Ezziddin et al. 2012).
Similarly, 86Y-DOTATOC has been used to assess the renal toxicity potential of 90Y-radio peptide therapy (Barone et al. 2005). As discussed above, 124I/131I is another example of a theranostic pair. Our group has utilized this approach with 124I-3F8 monoclonal antibody to predict dosimetry for 131I-3F8 treatment (Larson et al. 1992). Although there are some challenges associated with these non-standard positron tracers, only if we use such a strategy, can we exploit the true quantitative power of PET imaging.
It is currently standard practice with various pharmaceuticals to select candidates for therapy based on immunohistology (HER2 or genetic testing for EGFR mutations).
It is a widely accepted for all forms of targeted therapy, whether the therapy is pharmacologic with tyrosine kinase inhibitor drugs or radionuclide therapy with internal emitters, that patient selection is a key to success of therapeutic trials. This is the main benefit for theranostic applications in radionuclide therapy. It is envisioned that imaging methods may be substituted for these methods that are insensitive and may not give insight as to the heterogeneity of antigen/target distribution.
This is particularly true when it is possible to enrich patient population for subjects which are likely to respond to treatment with the specific targeting agent. As an example, 68Ga somatostatin receptor PET has been used to select patients and then to monitor response to treatment with 177Lu or 90Y labeled analogs. It is our opinion, that radio peptide therapy will be proven to be highly effective when theranostic quantitative imaging as a guide to patient selection is implemented on a broader scale in radio peptide therapeutic trials. There are also opportunities for combining radiopeptide therapy with chemotherapy in order to increase its efficacy. For example, a recent study combining radiosensitizing therapy with capecitabine and 177Lu-DOTA-TATE therapy has shown high response rates and tumor control rates. Figure 2 shows an example of a patient treated according to this protocol and demonstrates almost complete regression of the primary tumor and its metastases after two cycles of therapy.
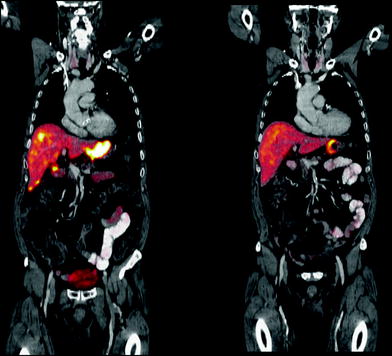

Fig. 2
68Ga-DOTA-TATE PET/CT before and after 177Lu-DOTA-TATE therapy showing almost complete regression of the primary tumor as well as hepatic metastases
4 Alpha Emitter Therapy in Oncology
Alpha emitters as therapeutic radionuclides for targeted radiotherapy may offer advantages in terms of therapeutic index for tumor treatment in certain key situations. This is because of the high linear energy transfer alpha radiation, which may be a thousand fold greater over shorter distances compared to corresponding beta emitting therapeutic radionuclides. Two clinical situations in which these advantages have been explored for targeted therapy in patients include bone metastases from prostate in an attempt to decrease skeletal-related events and improve survival, and in the treatment of leukemia.
4.1 Bone Palliation Therapy with Targeted Radionuclides
Pain from metastatic bone involvement with breast cancer, prostate cancer and lung cancer may be effectively treated with radionuclide targeting. Approved radiopharmaceuticals including 89Sr (Metastron©) and 153mSm-EDTMP (Quadramet©), are widely accepted and have been effective in reducing symptoms with minimum toxicity in advanced disease. Recently the radium 223Ra-dichloride has been introduced, for bone palliation therapy. The FDA has approved 223Ra-dichloride (Xofigo©) for the palliative treatment of castrate resistant prostate cancer metastatic to the bone, but not to the other organs. With this recent approval, 223Ra-dichloride has joined a handful of other drugs offering survival benefit in this patient group. ALSYMPCA trial, a phase III study was the basis of this approval which has shown statistically significant survival benefit for patients treated with 223Ra-dichloride plus standard of care versus standard of care alone. The current approved regimen for this radionuclide is 50 kBq (1.35 μCi)/kg body weight administered in 4 week intervals for six injections.
223Ra-dichloride has proved highly effective in control of pain, and in addition it has been associated with significant reduction in biomarkers, and as discussed above, also prolonged survival in patients with prostate cancer. The favorable toxicity profile of alpha emitters suggests that this form of therapy may also offer significant advantages in combination with chemotherapeutic agents commonly used in the treatment of advanced cancers. Because of the combination of effective control of pain, demonstrated antitumor response including biomarker reduction and improves survival, as well as the favorable toxicity profile, it is likely that 223Ra will be widely used in oncology as the agent of choice for treatment of bone pain in advanced metastatic cancers (Fig. 3).
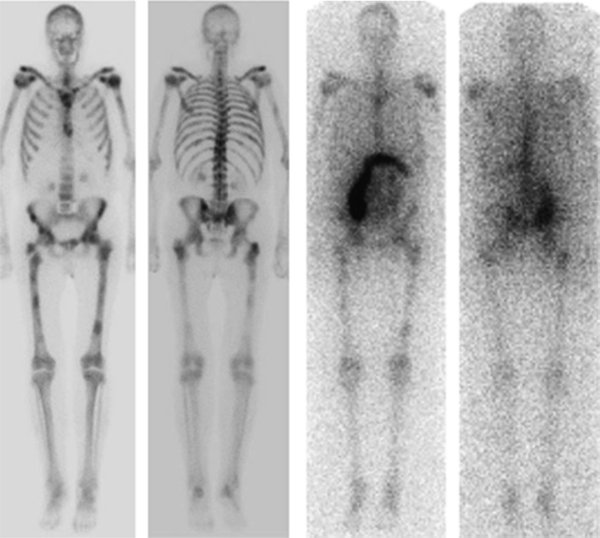

Fig. 3
Bone scans and Ra-223 dichloride scans of a patient with castration-resistant metastatic prostate cancer showing accumulation of Ra-223 in metastatic lesions. Ra-223 has been shown to undergo bowel transit and ultimately fecal excretion with minimal urinary excretion. Reprinted with permission from Springer©. Carrasquillo et al. Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra-dichloride in men with castration-resistant metastatic prostate cancer, 8 May 2013 (Epub ahead of print)
4.2 Augmenting the Therapeutic Armamentarium in Leukemia/Lymphoma with Radiolabeled Antibodies Including Alpha Emitter Antitumor Antibodies
So far two radiolabeled antibody formulations, Bexxar© (131I-tositumomab) and Zevalin © (90Y-ibritumomab tiuxetan) have been approved by FDA for use in therapy of hematopoietic malignancies. These formulations offer major advantages when used in the appropriate patient population. Details about these important forms of targeted therapy are provided elsewhere in this book.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree