Chapter 32 Hydrocephalus is one of the most common sequelae of any insult to a child’s central nervous system (CNS). Hydrocephalus occurs in 1 in 2000 live births and is associated with one third of all CNS malformations. Since the 1970s, the incidence of spinal dysraphism related to hydrocephalus has declined.1 Reasons include maternal folate therapy, which has resulted in fewer patients with spinal dysraphism, and vaccinations, which have diminished the number of patients with meningitis and its complications. The sites of CSF absorption remain controversial. It is widely accepted that arachnoid villi are one of the major sites in adults and older children.2 The arachnoid villi (pacchionian granulations) are not developed in children until the closure of the fontanels. Various studies also have suggested that a portion of CSF drains through the perivascular and perineural spaces into the lymphatic system.3 In neonates, most CSF absorption may occur through the lymphatic and venous system.4 The hydrodynamic model is based on the concept that the absorption of CSF occurs through the capillaries in the CNS rather than through the arachnoid granulations and villi.3 The skull is a nonelastic housing for brain tissue; blood, CSF, and brain tissue are almost incompressible. As stated by the Monro-Kelly doctrine, the total volume of arteries, veins, CSF, and brain confined within the skull cavity and dura mater is constant, and any increase in volume in one or more compartments causes a decrease in volume in the others. Skull and dura mater are more elastic. The elasticity of these structures plays a pivotal role in the hydrodynamic theory of hydrocephalus. During cardiac systole, the expansion of the intracranial arteries increases the ICP, causing CSF displacement into the spinal canal and an increase in the venous outflow. During cardiac diastole, inflow of CSF from the spinal canal occurs, which causes elevation of pressure in the subarachnoid space. Thus increased pressure is present in the CSF spaces during the entire cardiac cycle, which in turn compresses the venous outlets, causing an increase in outlet resistance and venous “counter” pressure. This pressure is necessary to keep the intracerebral veins sufficiently distended to accommodate the normal cerebral flow. Computed Tomography and Magnetic Resonance Imaging Computed tomography (CT) and MRI are used as primary modalities to assess ventricular size. Ultrasound of the head is used as the initial study in infants with macrocephaly. Several parameters can help differentiate between hydrocephalus and ex vacuo dilatation of ventricles from cerebral atrophy in infants (Box 32-1). The most reliable sign of hydrocephalus is enlargement of the anterior and posterior recesses of the third ventricle (Fig. 32-1); this phenomenon does not occur in ex vacuo ventricular enlargement. The disproportionate enlargement of the recesses occurs because the thin hypothalamus and cisterns surrounding these recesses provide relatively little resistance to expansion. In contrast, the body of the third ventricle is restricted by the rigid thalami, which provide more resistance to expansion. The anterior recesses (i.e., the chiasmal and infundibular recesses) expand earlier than the posterior recesses (i.e., the pineal and suprapineal recesses), which is best appreciated on midsagittal MRI.5 On axial CT, dilation of the anterior recesses of the third ventricle is detected when the third ventricle is larger at the level of the optic chiasm than at the middle of the ventricle. Figure 32-1 Obstructive hydrocephalus. Commensurate dilation of the temporal horns with the lateral ventricles also is a strong indicator of hydrocephalus. The dilation of the temporal horns is best viewed on coronal T2-weighted images. The choroidal fissure is enlarged, and the hippocampus is compressed and displaced inferomedially (Fig. 32-2). Studies have suggested that temporal horns dilate less than the bodies of the lateral ventricles in generalized atrophy.6 This finding may be related to the small size of the temporal lobes and to their relatively small volume of white matter. Figure 32-2 Magnetic resonance imaging findings in a patient with hydrocephalus. The mamillopontine distance is measured on MRI from the anterior root of the mamillary body to the top of the pons parallel to the anterior mesencephalon. The normal average distance is 3.8 mm.5 The floor of the third ventricle as seen on sagittal MRI is usually concave downward. With enlargement of the third ventricle, it becomes straightened or convex downward, resulting in reduction of the mamillopontine distance (e-Fig. 32-3). e-Figure 32-3 The mamillopontine distance. The ventricular angle (e-Fig. 32-4) measures the divergence of the frontal horns.6 Concentric enlargement of the frontal horns in a patient with hydrocephalus causes diminution of this angle, as seen on axial or coronal images. This concentric dilation produces an enlargement of the frontal horn radius with a rounded configuration of the frontal horns, or a “Mickey Mouse ears” appearance. e-Figure 32-4 Various methods of radiographically diagnosing hydrocephalus. The presence of periventricular interstitial edema is indicative of hydrocephalus (Fig. 32-5). With elevation of pressure within the ventricles, the normal centripetal flow toward the ventricles is reversed. The CSF is forced out through the ependyma into the surrounding extracellular spaces to be absorbed by alternative routes. This increase in periventricular fluid constitutes interstitial edema. It is best recognized on MRI with fluid-attenuated inversion recovery and proton density sequences. It is more difficult to appreciate on T2-weighted images because of the bright signal from the ventricles. Periventricular interstitial edema is difficult to appreciate in neonates and young infants because it is masked by a bright signal from immature myelin, with its high water content. On CT, periventricular interstitial edema is seen as hypoattenuation in the periventricular region, with indistinct ventricular margins. Figure 32-5 Hydrocephalus. A CSF “flow void” in the third ventricle, aqueduct of Sylvius, and fourth ventricle may be accentuated in persons with hydrocephalus as a result of hyperdynamic flow, although the specificity of this finding is unclear (e-Fig. 32-6). e-Figure 32-6 Rapid development of hydrocephalus. Marked hydrocephalus may lead to the formation of atrial diverticula, which is herniation of the ventricular wall through the choroidal fissure of the ventricular trigone into the supracerebellar and quadrigeminal cisterns. Diverticula may cause compression and distortion of the tectum and may mimic arachnoid cysts in the region of the quadrigeminal cistern.7
Hydrocephalus
Physiology of Cerebrospinal Fluid
Mechanisms of Hydrocephalus
Hydrodynamic Model for Csf Circulation
Imaging
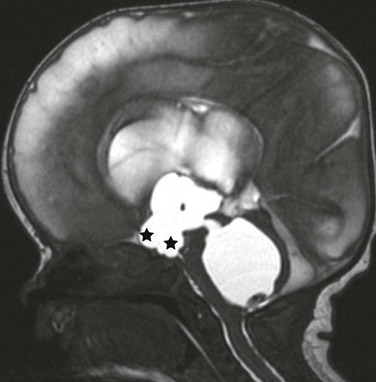
A midline sagittal balanced steady-state free precession image demonstrates the dilatation of the chiasmatic and infundibular recess of the third ventricle (stars). A dilated fourth ventricle also is noted in this patient with fourth ventricular outflow obstruction (an entrapped fourth ventricle) from neonatal hemorrhage.
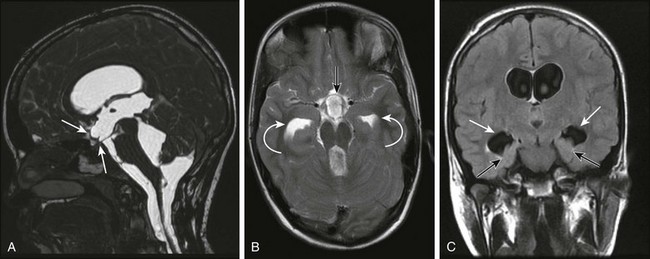
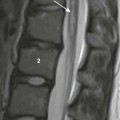
A, A midsagittal T1-weighted image in a 10-year-old girl with obstructive hydrocephalus demonstrates dilation of the chiasmatic and infundibular recesses (arrows). B, An axial fluid-attenuated inversion recovery image shows dilated anterior recess of the third ventricle (straight arrow). The temporal horns also are dilated (curved arrows), with a surrounding increase in signal suggestive of increased transependymal cerebrospinal fluid resorption. C, A coronal T2-weighted image shows the characteristic dilation of the temporal horns (white arrows) with enlargement of the choroidal fissure and inferomedial displacement of the hippocampus (black arrows).
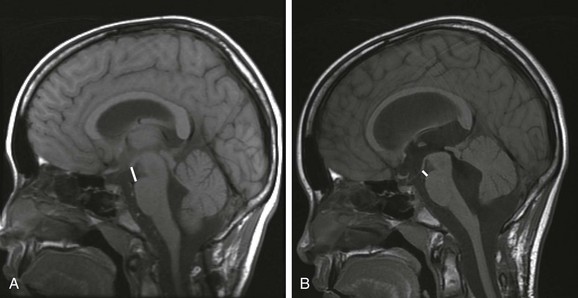
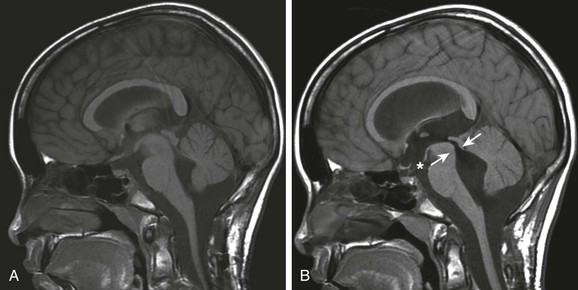
A, A baseline T1-weighted, sagittal, midline, magnetic resonance image in this 11-year-old patient demonstrates normal mamillopontine distance. The distance (bar) is measured from the anterior base of the mamillary body to the top of the pons parallel to the anterior aspect of the mesencephalon. B, A reduction in the mamillopontine distance within 6 months as a result of interval development of hydrocephalus from an intraventricular hemorrhage. Note the stretching of the corpus callosum and the third ventricular dilation.
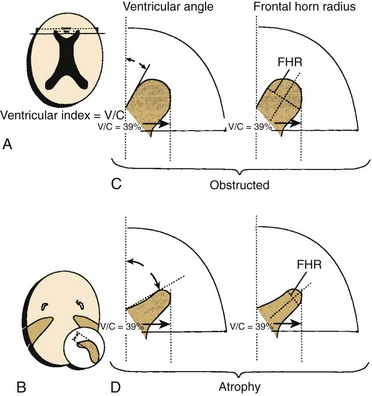
A, The ventricular index is the ratio of the ventricular diameter at the level of the frontal horns to the diameter of the brain measured at the same level. This method is not very sensitive because the ventricular index is enlarged in patients with cerebral atrophy, as well as in patients with hydrocephalus. B, The finding that the temporal horns have enlarged commensurately with the bodies of the lateral ventricles is probably the most sensitive and reliable sign in the differentiation of hydrocephalus from atrophy. There is significantly less dilation of the temporal horns than of the bodies of the lateral ventricles in cerebral atrophy. C, The ventricular angle measures the divergence of the frontal horn. In theory, the angle made by the anterior or superior margins of the frontal horn at the level of the foramina of Monro is diminished when concentric enlargement of the frontal horns occurs. Compare the illustration of hydrocephalus (top) with that of atrophy (bottom). The ventricular index in both cases is 39%, but the ventricular angle is markedly reduced in the presence of hydrocephalus. D, Concentric dilation produces an enlargement of the frontal horn radius (FHR) with a rounded configuration of the frontal horns, or a “Mickey Mouse ears” appearance. (From Barkovich AJ. Pediatric neuroimaging. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2005.)
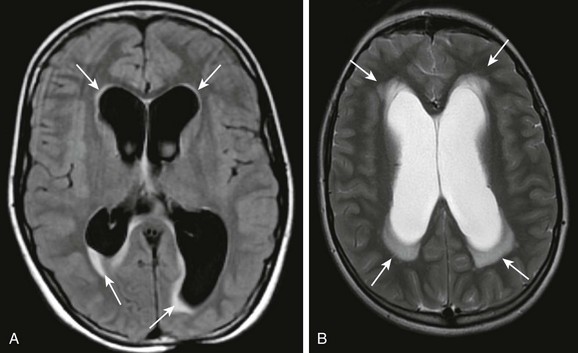
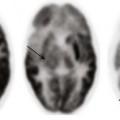
A, An axial fluid-attenuated inversion recovery sequence at the level of the lateral ventricles demonstrates characteristic transependymal flow of cerebrospinal fluid (CSF) and periventricular hyperintensities. B, An axial T2-weighted image in a different patient demonstrates increased transependymal flow of CSF characteristic of hydrocephalus.
A, Imaging at baseline: a T1-weighted midsagittal image shows normal anatomy. B, Imaging at 1 month follow-up: a flow void in the aqueduct of Sylvius indicating increased CSF velocity (arrows) is noted with enlarging lateral ventricles (not shown), indicative of hydrocephalus. Also note the dilatation of the anterior recesses of the third ventricle (asterisk).
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Hydrocephalus