Yann Lachenal • Alban Denys • Pierre E. Bize
BACKGROUND
With the increasing number of minimally invasive diagnostic and therapeutic procedures, iatrogenic complications have become more frequent.1–6 These complications can have no clinical impact and remain silent but can also have dramatic outcomes. Transarterial embolization (TAE) is in first line for treatment of vascular lesions such as active hemorrhage, pseudoaneurysm (PA), arteriovenous fistula (AVF), and arteriocavitary fistula. It is then of the highest importance for the interventional radiologist to know them, recognize them and be able to manage them.2,5–12
Iatrogenic Vascular Access Lesions
The common femoral artery is the most frequent arterial access of vascular procedures, with an overall complication rate around 6%.13 Other vascular access locations are less used and have a complication rate estimated from 2% to 36% for brachial access,14–16 7.7% to 15.7% for popliteal access,17–19 and less than 3% for transradial access.13 The most frequent vascular complications are hematoma, AVF, PA, distal embolization, dissection, and thrombosis.2 There are different ways to manage common femoral artery access–related injuries, but the discussion will focus on iatrogenic lesions that require embolization.
Iatrogenic Renal Lesions
Iatrogenic traumas are the main cause of transplanted and native kidney vascular injuries.20–23 Hemorrhagic complications are frequent, with perirenal hematoma larger than 2 cm seen in 12.5% and macroscopic hematuria seen in 3.8% of patients after percutaneous biopsy.24,25 Because of their prevalence, percutaneous biopsies are the leading cause of iatrogenic vascular injury in kidney, despite reported rate of transfusion and embolization in recent studies of 0.9% and 0.2% to 0.6%, respectively.25,26 Major hemorrhagic complications requiring transfusions and/or embolization are seen in 12.5% of transjugular kidney biopsy,27,28 1% to 4% of percutaneous nephrostomy,29 12% to 14% of percutaneous lithotripsy,29 6% of partial nephrectomy,30 5% of radiofrequency ablation,31 and 0.7% to 2.6% of cryoablation.32,33
Iatrogenic Liver Lesions
Iatrogenic traumas are currently the main etiology of hemorrhagic complications seen in the liver. This situation finds its origin in the increasing number of percutaneous procedures over the last few decades.1,6 Major hemorrhagic complications have been described after percutaneous (biopsy, percutaneous transhepatic cholangiography [PTC], percutaneous transhepatic biliary drainage [PTBD], radiofrequency ablation [RFA]), endovascular (transjugular hepatic biopsy, transjugular intrahepatic portosystemic shunts [TIPS]), endoscopic (retrograde cholangiography, endoscopic retrograde cholangiopancreatography [ERCP]), and laparoscopic (cholecystectomy) interventions as well as open surgery (pancreatobiliary surgery, liver transplantation) with an incidence of 0.3% to 6%.1,8,34–43
Arterioportal fistula (APF) is the most frequent arterial injury type. Iatrogenic injuries are mostly intrahepatic.43,44 The rate of APF is 4.2% in the first week following liver biopsy.45 Most seem to close spontaneously, whereas only a minority progresses.45–47 Clinically, only 17% of APF are symptomatic,44 with findings related to hemobilia and portal hypertension, such as gastrointestinal bleeding, ascites, abdominal pain, and diarrhea.48,49 Diagnosis and management are decided after Doppler ultrasound (US) examination (see the section “Clinical Applications”).
Arteriohepatic vein fistulas (AHFs) are rare and not well studied. Their finding is often incidental on computed tomography (CT) in patients who had a liver biopsy in the past. AHFs may lead to high-output cardiac failure by increasing cardiac load, but they usually stay clinically silent as vascular shunt is insignificant.43,45
PA of hepatic artery is usually of iatrogenic origin. Percutaneous procedures are the leading cause of intrahepatic lesions, whereas endoscopic procedures, laparoscopy, hepatobiliary surgery, and liver transplantations are the cause of extrahepatic lesions.43,50 The right hepatic artery is the most common artery involved.50
Iatrogenic Splenic Lesions
Most splenic injuries are caused by trauma or pancreatitis. Iatrogenic lesions contribute only to a minority of cases and are caused by splenic biopsy and abdominal surgery.51 Incidence of major complications after splenic biopsy is 2.2%, most of which are hemorrhagic.52 The rate of splenic injuries during abdominal surgery is 0.5%.53 These lesions are usually recognized and treated surgically immediately. Therefore, the incidence of splenic injuries not recognized during surgery that would require TAE is not known.54 The spectrum of arterial injuries is the same as for blunt trauma: active bleeding, PA, and AVF. Clinical presentation is variable and extends from absence of symptoms to hemodynamic collapse. AVF can additionally show symptoms of portal hypertension such as abdominal pain, gastroesophageal varices, and intestinal bleeding.55
Iatrogenic Pulmonary Lesions
Hemorrhagic complications are seen in up to 23% of lung biopsies,56 but massive hemorrhages are encountered in less than 0.5% of patients after biopsy35,57 and less than 1% after RFA.58,59 Hemoptysis is the main clinical manifestation.Mortality after biopsy is low (0.07%)57 and is due rather to asphyxia than to exsanguination when caused by lung hemorrhage. Hemorrhagic lung complications are often self-limiting and usually do not require endovascular treatment. Lesions to the pulmonary artery with formation of a PA after Swan-Ganz catheter placement and RFA have been described and treated successfully with embolization, but these lesions remain rare.58–60
DEVICE/MATERIAL DESCRIPTION
The choice of embolic agent that will be used depend mostly on the type of lesion that will be treated and on operator preference. Gelatin sponge is effective and inexpensive. Vascular occlusion is transitory and lasts a few days with progressive recanalization of occluded artery. This property is its main drawback, because if healing of the arterial injury is not complete, bleeding can reappear. N-butyl cyanoacylate (NBCA) is effective and permanent. Occlusion is immediate. Because of its liquid nature, it flows through vessels with blood and occludes vessels more distally than coils. In liver and other organs, this property is useful if catheter access is limited. Drawbacks are the long learning curve to use glue and the risk of nontarget embolization. Coils are very effective and provide a permanent occlusion. However, they can’t be positioned as far as liquid agents. Covered stents are used to treat arterial parietal injuries without occluding the feeding artery. Often, materials are mixed to increase embolization efficacy. Situations in which these various embolic agents should be used will be discussed in more detail in the “Clinical Applications” section.
TECHNIQUE
Iatrogenic Vascular Access Lesions
PAs are not usually treated by TAE but rather by US-guided compression, direct thrombin injection (see “Tips and Tricks”), stent graft insertion, or surgical repair. AVFs are not usually treated by embolization but by US-guided compression, direct thrombin injection, or stent graft placement over the neck of the fistula. In this case, stent diameter should be oversized 1 mm larger in normal artery and 2 mm in calcified artery.2 Retroperitoneal hemorrhage can usually be treated with a stent graft when it is caused by a leak from a major vessel such as the common femoral or the external iliac artery. The stent graft diameter should be oversized as stated earlier. When the bleeding occurs from a secondary branch (as the circumflex iliac artery) usually perforated by an inappropriate guidewire manipulation, this secondary branch can usually be embolized with coils, Gelfoam (Ethicon, Johnson & Johnson, Somerville, New Jersey) or a mix of them.
Iatrogenic Renal Lesions
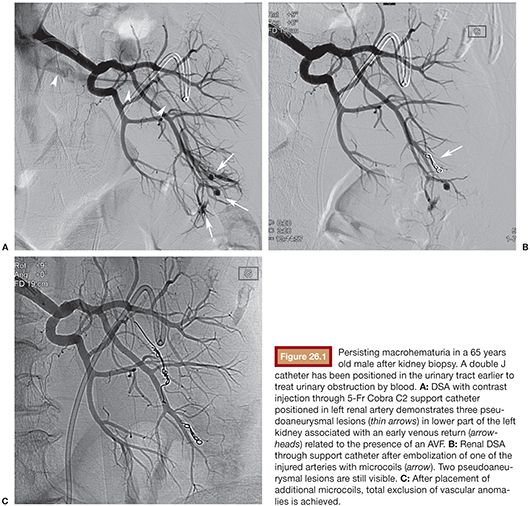
Catheterization of the renal artery is performed with a Cobra, Renal Double Curve (RDC), H-Stick, or Simmons 5-Fr support catheters (Cordis, Johnson & Johnson Medical, Waterloo, Belgium). Catheter choice depends on the orientation of the renal artery. Glide catheters should be avoided as they do not provide strong support. Images can show active retroperitoneal or calyceal hemorrhage, arterial transection, PA, AVF, or deformation of renal parenchyma by subcapsular or perirenal hematoma. Iatrogenic lesions are usually unifocal, so superselective catheterization with 3-Fr or less coaxial microcatheter is the rule. PA should ideally be embolized with microcoils filling the PA and extending proximally in the afferent artery. When selective catheterization is not feasible, embolization of afferent artery is enough. The classical technique of PA embolization using sandwich technique is not necessary as kidney vascularization is terminal with few collaterals. NBCA can be used to fill PA and extend in the feeding artery. It should be used if lesions are multiple or too distal in location to be reachable with a microcatheter.10 Gelfoam alone should be avoided as it resorbs over a few days, but it can be mixed with coils to improve efficacy of embolization. AVF embolization is most often performed with microcoils (Fig. 26.1). Care must be taken in the choice of type and size of coils as there is a risk of coil migration and nontarget embolization. They must be oversized 1.5 times the diameter of the target vessel. For high-flow fistulas, the first coil should be detachable and have enough length for a good control of its stability before its release and to build a frame. Afterward, pushable coils of smaller size are used to fill the frame. NBCA can be used, with usual caution. Gelfoam or particles should be avoided. Vascular plugs are sometimes used, but their delivery may be impossible in tortuous vessels. In case of arteriocalyceal fistula with active hemorrhage, embolization of feeding artery with microcoils or NBCA is usually efficient.
Iatrogenic Liver Lesions
Single curve, Cobra, Sidewinder, or Simmons 5-Fr support catheters (Cordis, Johnson & Johnson Medical, Waterloo, Belgium) are used to access celiac trunk. Catheterization should be delicate to avoid dissection. After a digital subtraction angiographic (DSA) serie by support catheter with patient in apnea, navigation to the target lesion is performed with a 3-Fr or less coaxially inserted microcatheter. DSA may show PA, APF, arteriobiliary fistula, artery transection, and active bleeding.
Hepatic PA embolization approach depends on location of vascular injury.
If the PA is extrahepatic (common hepatic artery, proper hepatic artery and its right and left branches), exclusion can be performed with a covered stent 4 to 6 mm diameter depending on artery size (Coronary Graftmaster covered stent [Abbott Vascular, Diegem, Belgium] or Advanta V12 covered stent [Atrium, Rastatt, Germany]) or by filling the lesion with coils or NBCA to preserve permeability of the injured artery. This approach is mandatory in transplanted livers as they are very sensitive to ischemia. However, sacrifice of the left or right branch of the hepatic artery can be considered in native liver, because intrahepatic collaterals from one side to the other open immediately after vascular occlusion and oxygenation by portal vein is usually sufficient if this vein is patent and that portal venous flow is not hepatofugal.
If the vascular injury is intrahepatic, treatment depends on selectivity of microcatheterization. If possible, microcoils or NBCA should extend across the PA’s neck in distal and proximal artery to avoid backflow vascularization of the PA by collaterals. Catheterization of the PA itself should be avoided because of the risk of rupture by microcatheter tip. To facilitate stasis and thrombosis, Gelfoam can be used between coils (“sandwich technique”) or to fill the PA feeding vessel. As the PA does not have walls, a basic principle is to avoid treating the PA sac like a true aneurysm would be treated. Attention should be paid to embolize the feeder(s) vessel(s) if possible. If only the PA sac is embolized (the exception is embolization of common femoral artery with direct thrombin percutaneous injection), there is a tendency to recanalization and reexpansion of the PA sac around the embolic agent as the feeder vessel can transmit pressure to the soft tissues surrounding the PA. If microcatheter positioning close to the lesion is not possible, Gelfoam or NBCA should be used in association or not with coils. Finally, direct percutaneous puncture of the PA with injection of thrombin or coils has been described for unreachable lesions.43,61
Embolization of APF should be performed with microcatheter closely positioned to the fistulous site. Embolization is usually made with coils, but other embolic agents have been used (detachable balloon, NBCA, Onyx [EV3 Europe SAS/International, Paris, France], microspheres [Bead-Block, Biocompatibles, Farnham, UK] and Gelfoam).62 The fistulous tract is sometimes very short and it becomes unavoidable to embolize the feeding artery, so great caution should be taken before embolization of a lesion in an operated or transplanted liver because of the risk of ischemia. Decrease of flow through fistula rather than complete occlusion is sometimes enough to prevent the progression of APF.43 Deployment of coils must be done carefully because of risk of migration.
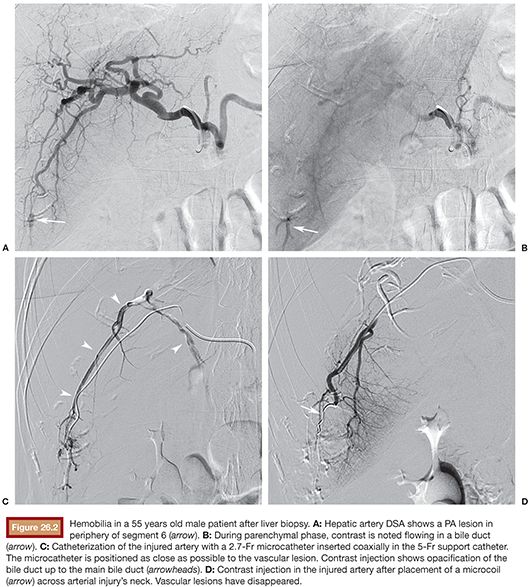
The approach to arteriobiliary fistula or artery transection with active bleeding is the same as for PA (Fig. 26.2).
Iatrogenic Spleen Lesions
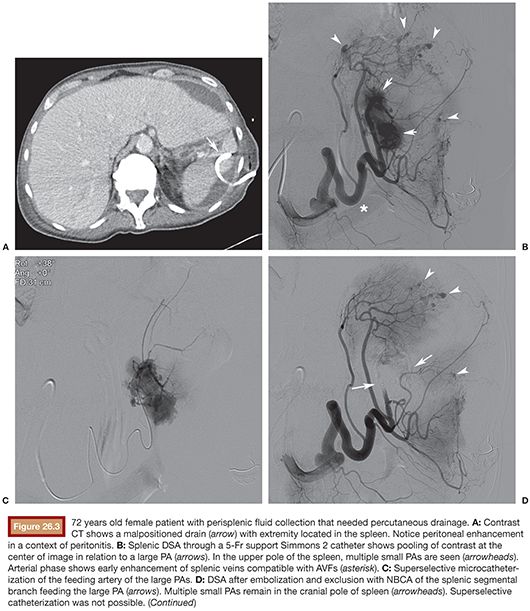
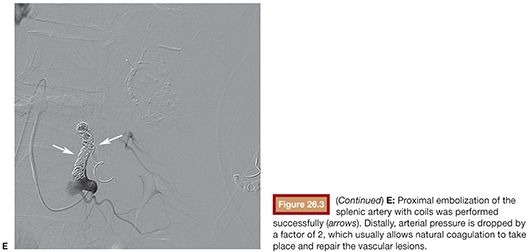
Access to celiac trunk is performed with 4-Fr or 5-Fr support catheter, usually a Cobra C2 or a Simmons 1 or 2. If possible, direct catheterization and DSA of splenic artery should be performed first to save time and contrast. A vascular lesion might appear as a PA, AVF, contrast extravasation, artery transection, or splenic parenchymal deformation by hematoma. Number, topography, and accessibility of lesions are assessed to determine indication for proximal and/or distal splenic artery embolization (see the section “Clinical Applications”). A 3-Fr or smaller microcatheter is coaxially inserted to access embolization chosen site selectively. Splenic artery diameter usually measures 7 to 8 mm, so proximal embolization of the main splenic artery should be performed with 10- to 12-mm diameter coils. Attention should be paid that hypovolemic patients have spastic arteries, which can lead to underestimation of true lumen diameter. The superior polar splenic artery can have a proximal origin and should be checked for hemorrhage. Coils should be deployed between dorsal and magna pancreatic arteries to keep collateral flow to spleen. Anchoring of the first coil in a small branch is useful to avoid coil migration. The first coil must be long enough (usually 10 to 14 cm long) to build a solid frame before packing with smaller coils. The end point is stagnation of contrast upstream in splenic artery. For distal embolization of the splenic artery, a 3-Fr or smaller microcatheter is positioned as close as possible to the target lesion. PA should ideally be embolized “front door and back door” with embolic material (coils or NBCA) covering its neck. As intrasplenic collaterals are not numerous, if catheterization of the “back door” is not possible, embolization of the “front door” artery is often efficient and enough, especially if liquid embolic agent such as glue is selected. Catheterization of the PA itself should be avoided to minimize rupture risk. AVF are usually embolized with coils positioned in the fistula or just proximal to it. Care should be taken to avoid coil migration in venous flow. Active hemorrhage should embolized with coils, NBCA, or Gelfoam. For multiple lesions in the same splenic segment, segmental artery embolization with NBCA, gelfoam slurry, or coils is indicated. For lesions that are in different segments, superselective embolization can be performed if time and access are granted; otherwise, proximal embolization is efficient (Fig. 26.3). PVA particles should usually be avoided as they may cause profound parenchymal ischemia.
Iatrogenic Pulmonary Lesions
Because they are usually self-limiting, hemorrhagic complication of lung biopsies are often treated conservatively. To avoid filling of the airways with blood, patients presenting with hemoptysis after lung biopsy should be laid on the punctured side. Close monitoring of blood oxygen level should be performed as the main risk for these patients is suffocation. If blood oxygen level drops, cleaning of the airways with rigid fibroscopy should be performed promptly. Pulmonary artery PA from Swan-Ganz insertion or RFA can be treated by coil embolization from a venous peripheral puncture.
CLINICAL APPLICATIONS
Iatrogenic Vascular Access Lesions
Patient-related risk factors for vascular complications are hypertension, female gender, emergency procedures, high bifurcation of femoral artery, and anticoagulation. Technical risk factors are left groin puncture, method of puncture, arterial entry site, size of sheath, anticoagulation, and use of closure device.11,63–65 Hematoma of the groin is usually self-limiting. Small hematomas are frequent, but in up to 2.8% of patients, blood transfusion or invasive treatment is required.65 Clinically, immediate or delayed tumefaction appears around the arterial puncture site and increases the diameter of the thigh. Rarely, hematoma is hard to control even with manual compression. US or angio-CT is needed to exclude PA.
Pseudoaneurysm
PAs are seen in 1.2% to 8% of femoral punctures.63,66 Clinical manifestation is a palpable, painful, pulsatile, or thrilling mass, which can grow over time. Complications related to PA are rupture, distal embolization of thrombus, infection, skin necrosis, and nerve and vessel compression.67 Diagnosis is confirmed with Doppler US.11 In symptomatic patients, PA must be treated quickly. But in asymptomatic patients, it has been shown that most PAs less than 1.8 cm diameter spontaneously thrombose in less than 2 months.68,69 PA bigger than 1.8 cm and PA in anticoagulated patients, regardless of the size, must be treated.68,69 Due to its noninvasiveness, US-guided compression is considered as the first-line treatment. US-guided thrombin injection can be a valuable adjunct to US-guided compression (see “Tips and Tricks”) and has a success rate of 90% to 100%.2 However, surgery remains indicated in case of failure of other techniques.
Arteriovenous Fistulas
AVF between the femoral artery and vein is seen in less than 1% of inguinal punctures.63,64 The risk increases with low femoral artery puncture. AVFs are rarely symptomatic, but they can present with high-output cardiac failure, limb edema, claudication, or aneurysmal dilatation of femoral and iliac artery and veins. Diagnosis is confirmed by Doppler US.11 AVF should be treated because they tend to increase with time and can cause complications such as venous hypertension in the affected limb or high-output cardiac failure.2 They are usually treated by US-guided compression as first-line treatment. Covered stent placement and surgical repair are options in case of failure. There is concern about long-term patency of those stents and their resistance in a flexure point such as the groin, so this option might be interesting in older patients and in patients with contraindication to surgery, but surgical repair is preferred for younger patients.
Retroperitoneal Hemorrhage
Retroperitoneal hemorrhage occurs generally when arterial puncture is performed above the inguinal ligament. This complication can be life-threatening as there is no exteriorization of blood. Clinical signs (tachycardia, hypotension, abdominal pain, confusion, agitation) might appear only late after intervention. Incidence is low, less than 1% of femoral punctures. Noncontrast CT must be performed to make diagnosis and guide treatment.11
Retroperitoneal bleeding is usually self-limited, but in some cases, such as in patients with toubled coagulation status, it should be treated if size of hematoma is increasing.
Iatrogenic Renal Lesions
Clinical manifestations are related to hemodynamic consequences of blood loss and urinary obstruction: hematuria, flank pain, and hypovolemia. AVF can also lead to progressive renal failure or to new or uncontrollable hypertension as a consequence of blood steal and relative ischemia of renal parenchyma. Cardiac failure caused by high-flow shunting is also possible.70,71 These symptoms may be delayed for years after an intervention.72,73 Lesion types are artery transection with active bleeding, PA, arteriocalyceal fistula, AVF, or an association of these.23 Superselective embolization is the treatment of choice. The procedure is safe and effective, with a technical success rate of over 90% and clinical success rate over 80%.20,22,23,74–79 Surgery remains indicated in case of failure of endovascular treatment. The right timing for embolization is unclear. Hemorrhage is usually self-limiting. For mildly symptomatic, stable, and well-responding transfused patients, it is acceptable to wait 72 hours after intervention before going to embolization. For persisting or uncontrollable hematuria, retroperitoneal hemorrhage, deteriorating renal function, and unstable patients, emergency embolization is required.74,76,80 CT with contrast and US can categorize lesion types and help to plan embolization but are not mandatory before angiography. The approach is different in asymptomatic patients. Some older series with a limited number of patients have reported a spontaneous closure of AVF up to 75% and of PA up to 100% a few weeks postintervention.81–84 The risk of spontaneous enlargement and rupture of AVF and PA, and the fact that TAE is a safe procedure, should make this technique the first-line treatment in the management of these types of lesions.74
Iatrogenic Liver Lesions
Clinical manifestations include hemobilia, systemic hypotension, gastrointestinal bleeding, and perihepatic hematoma.1,8 Hemobilia has 94% positive predictive value for arterial injury1 and is the most frequent clinical sign, but its classic triad of abdominal pain, jaundice, and gastrointestinal bleeding is present in only 22% of patients.6 Clinical presentation may be delayed from days to months after intervention, but approximately 80% of vascular complications are discovered within 2 weeks after an intervention.8 At laboratory, arterial injuries should be suspected particularly if there is a 5% decrease of hematocrit level or abnormality/worsening of the hepatic tests after the intervention.1,48 It should also be noted that a large proportion of vascular injuries may remain clinically silent.6,45 TAE is currently the treatment of choice for these lesions, with a technical and clinical success rate of 75% to 100%.8,10,42,48,85–87 Surgery remains available in cases of failure of TAE, but has a higher morbidity and mortality. Embolization in transplanted liver and after pancreatico-biliary operation should be evaluated very carefully as liver are and particularly bile ducts become very sensitive to ischemia, as they are primarily vascularized by hepatic arteries.48
Angiographic findings are active bleeding, PA, APF, AHF (rare), arteriobiliary fistula, and nonspecific lesions.45 Complex injuries are defined by a mix of more than one lesion type.88 Symptomatic APF requires embolization. Estimation of the hemodynamic consequences of the fistula with Doppler US should be made.43,48 A significant shunt is defined by a low arterial resistive index (RI) or a drop of RI ≥ 0.10 compared to previous examinations and by reversal of flow in portal vein or first-order branch, with or without arterialization of spectral flow.43,48 Angiography confirms presence and estimates shunt during intervention. APF is considered significant if contrast flows back to portal vein or first-order branch.44,49 In conclusion, in asymptomatic patients with large and hemodynamically significant APF, embolization is required, whereas in others, surveillance is probably enough.43,44,48,49 Hepatic artery PAs have a reported rupture rate50 as high as 76% and a mortality rate of 16% to 43%.50,89 In consequence, treatment is required regardless of symptomatology. However, in transplanted liver and after pancreatico-biliary surgery if PA is small, asymptomatic, and nonevoluting, a conservative attitude may be indicated.43 Arteriobiliary fistulas are managed as PA.
Iatrogenic Spleen Lesions
Splenic TAE has a high technical success rate close to 100% and a high clinical success rate of around 91.1%.12 The natural evolution of splenic PA is unclear. It appears that an unknown proportion of PA spontaneously thromboses.51,90,91 As PAs have a high rupture and mortality risk, recommendation is embolization of all PA independently of symptomatology.51 The spontaneous evolution of AVFs is also unclear. It seems logical to embolize them, unless they are present for a long time and are asymptomatic.
Regardless the lesion types, proximal embolization of the splenic artery is indicated when the splenic artery is too tortuous to allow microcatheterization of the spleen, when bleeding is diffuse or multifocal, or when hemodynamic instability is severe and time is lacking. Distal embolization is indicated when bleeding is unifocal or paucifocal.92 After proximal TAE of the splenic artery, the spleen remains vascularized through short gastric, gastroepiploic, and pancreatic collaterals, but pressure in the splenic artery is reduced by a factor of 2, allowing coagulation to occur naturally.93–95
COMPLICATIONS
Iatrogenic Vascular Access Lesions
Thrombus leakage during thrombin percutaneous injection through PA neck with distal nontarget embolization is a known complication. So, in lesions with wide neck, a balloon can be used to cover the PA neck during thrombin injection (see the section “Clinical Applications”). In case of AVF, embolization material may flow directly to the right atrium and lungs or even the left atrium in case of patent foramen ovale. This is why US-guided compression, surgical repair, and, in selected patients, covered-stent placement is preferred when neck is wide. Stent graft deformation and fracture can occur if deployed in flexure points. Care should also be taken before stent graft deployment because of risk of occlusion of unwanted vessels (typically deep femoral artery).2
Iatrogenic Renal Lesions
Ischemic parenchymal territory after embolization is estimated from less than 50% in older series23 to less than 10% in recent ones.76 Moreover, there is a partial reperfusion of the ischemic territory after some time due to collateral vascular supply.20,76 Studies have failed to demonstrate any effect of embolization on worsening of renal function and blood pressure.20,21,74,75,77–79,96,97 Other complications are nontarget embolization, coil migration, artery dissection, thrombosis, postembolization syndrome, abdominal compartment syndrome, and renal abscess.98
Iatrogenic Liver Lesions
Usually, the complication rate after TAE in the liver is low because of the dual vascularization of liver by portal vein and hepatic artery. Moreover, intraparenchymal arterial collaterals are opening immediately after embolization.99 So, in the native liver, parenchymal necrosis is rare, occurring only in 4.2% of patients after TAE.8 Transitory ischemia of liver parenchyma is reflected by transient increase of liver enzymes and explains part of the postembolization syndrome, which is seen in 20% of patients.3,8,42 Patients who have undergone pancreatic, biliary, or hepatic surgery or liver transplantation, as well as those with underlying liver disease, have a higher risk of liver necrosis and failure.100 Embolization is contraindicated in patients with portal vein thrombosis because of the risk of ischemia.44 As the bile ducts are vascularized by the hepatic artery, ischemia after TAE may lead to wall necrosis with stenosis or fistulas. As consequence, there is an increased risk of biloma, cholangitis, hepatic abscess, or peritonitis.5 A small series shows 6.7% hepatic abscess formation after TAE for hepatic arterial injuries.87 Nontarget embolization may lead to gallbladder, pancreas, or spleen ischemia or inflammation.
Celiac trunk and hepatic artery dissection with or without occlusion may occur, especially in patients with arcuate ligament syndrome and fibromuscular dysplasia, both infrequent entities.
Iatrogenic Spleen Lesions
Postembolization syndrome occurs in 30% of patients with fever, abdominal pain, slowed transit, and sometimes pancreatitis.101 Other complications such as abscess formation, spleen infarction, and abnormal fluid collections are seen in 3.8% to 7%, 10% to 43%, and 43% of patients, respectively.12,102–104
TIPS AND TRICKS
Minimally Invasive Treatment of Vascular Access Complications
• US-guided compression of PA: The probe is positioned over the neck of the PA and a pressure is applied until flow disappearance inside the PA. Pressure is maintained until complete thrombosis. This method is not invasive and has few complications but also has drawbacks. It takes time as the compression should be applied during at least 20–30 min and can be very painful; the failure rate is between 30% and 40% in anticoagulated patients.105 Strong analgesia with intravenous opioids should be considered.
• Direct percutaneous thrombin injection: Under US guidance, a small-diameter needle (19–21 gauge) is inserted in the PA and positioned at its center. Color Doppler US should show the needle tip in the part of the PA with blood flowing away from the neck to minimize thromboembolic risks. 500–1,000 International Units of thrombin should be injected in small pushes. This dose is usually enough to completely embolize the PA. If the PA is multiloculated, embolization should begin with the farthest loculus from PA neck. The coagulation status of the patient does not seem to affect thrombin injection efficacy. The risk of thrombin leakage in the femoral artery and distal embolization is low.106 To protect the limb from distal thromboembolic event, in PAs with large and short neck or complex PA, an occlusion balloon can be inserted from a contralateral arterial access. It should be inflated to occlude the neck of the PA during thrombin injection.107 The balloon should stay inflated for 10–15 min afterward. US Doppler should be used to confirm disappearance of flow in the aneurysm before and after thrombin injection. Neck width larger than 3 mm in diameter has been proposed as the minimal diameter for this technique (Fig. 26.4).108
Renal and Splenic Pseudoaneurysms
• PA in the renal and splenic vasculature can usually be treated with proximal embolization only because parenchymal vascularization is almost terminal with few collaterals.
Arterial Embolization in the Liver
• Before performing embolization in the native liver, care should be taken to verify permeability of the portal vein and direction of the portal vein flow because of the risk of ischemia. It is particularly important in liver to embolize front door and back door vessels because liver has many intraparenchymal collaterals and there is a risk of recanalization of lesion.87 Embolization with coils or thrombin of a PA through a direct percutaneous transhepatic approach has been described in cases where TAE was not feasible.109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree