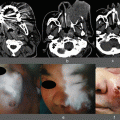
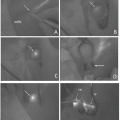
Fig. 36.1
(a) and (b): Ordinary view and fluorescence image obtained at the time of initial insertion of the infrared laparoscope
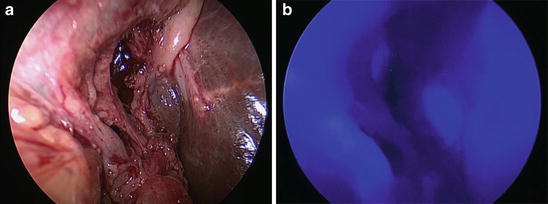
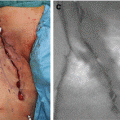
Fig. 36.2
(a) and (b): Fluorescence imaging after identification of the cystic duct and artery
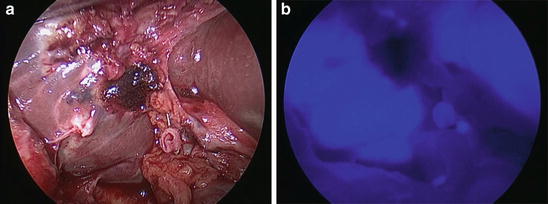
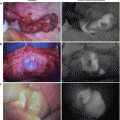
Fig. 36.3
(a) and (b): We confirmed no bile leakage from the stump of the cystic duct
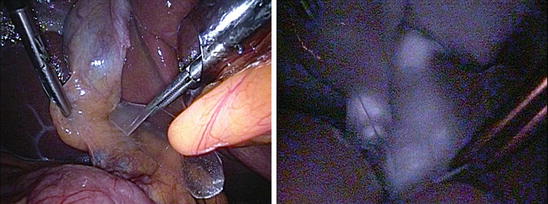
Fig. 36.4
(a) and (b): Clearer fluorescence imaging over the biliary tract obtained after compression of TFPD
36.3 Results
There were no conversions to open cholecystectomy. The mean operation time was 83 min (range, 46–163 min), and the estimated intraoperative blood loss was minimal. The fluorescence imaging over the biliary tract including common hepatic duct, common bile duct, and cystic duct was clearly obtained through the laparoscope in all patients. However, gallbladder could not be clearly visualized due to inflammation, edema, or an impacted stone in the neck of the gallbladder. Local compression by our original TFPD provided a clearer fluorescence image of the biliary tract against dense adipose tissues. In particular, the identification of the confluence between the cystic duct and the common bile duct was important to perform the safe procedure. The location and blood flow within the cystic artery were identified approximately 10 s after reinjection of ICG (2.5 mg/body) intravenously. However, an identification of cystic artery also depends on the underlying conditions of the patients. There were no adverse events related to the administration of ICG. The mean postoperative hospital stay was 3 days (range, 2–4 days), and there were no postoperative complications during the follow-up period.
36.4 Discussion
ICG is stabilized in plasma protein after intravenous injection, and protein-bounding ICG emits light with a peak fluorescence wavelength of 845 nm when exposed with near-infrared light with a wavelength of 760 nm. ICG is known to be safe at the doses used in routine clinical practice, and the risk of adverse events is quite low (approximately 0.003 %) at doses exceeding 0.5 mg/kg [8]. Indeed we administered ICG at only 1 ml/body (0.04–0.05 mg/kg) at a concentration of 2.5 mg/ml for intraoperative exploration of the biliary tract. In the literature, there are no reports of adverse events related with intravenous injection of ICG in hepatobiliary surgery [6–8, 10–16].
BDI during cholecystectomy is a serious surgical complication that brings poor quality of patient’s life. Törnqvist et al. [5] reported that an early detection of BDI during primary surgery improved survival, and the intention to use intraoperative cholangiography (IOC) reduced the risk of death after cholecystectomy. Therefore, an intraoperative modality for the detection of the biliary anatomy is an important factor to obtain a safe outcome. Although IOC via cannulation of the cystic duct during cholecystectomy was a standard and routine method for obtaining the information of the biliary system including bile leakage or common bile duct stone up to date, recently, near-infrared fluorescence imaging with ICG has been penetrated as an intraoperative assessment for an identification of biliary anatomy in several institutions [6, 10–12]. Fluorescence imaging realized a real-time observation, noninvasiveness, and easy performance compared with ordinary intraoperative cholangiography and became an attractive tool for identification of biliary anatomy including biliary tract and hepatic arteries.
To obtain an adequate fluorescence imaging during cholecystectomy, the injection timing of ICG is a very important factor. From the literature, Matsui et al. [17] reported that the time interval between intravenous injection and the recognition of extrahepatic bile duct with florescence imaging was quantified at 90 min in pig models, Aoki et al. [11] reported that satisfactory results were obtained by the administration of ICG 30 min or 1 h before surgery in human study, and Schols et al. [14] reported that ICG was visible in the liver and bile ducts within 20 min after injection. Ishizawa et al. [18] reported that the time interval before dissection of the triangle of Calot ranged from 35 to 75 min. Our time interval using pig models was approximately within 20 min after intravenous injection; however, fluorescence imaging of the gallbladder required further 30 min. Therefore, we have routinely injected ICG 1 h before surgery to observe an adequate fluorescence imaging from the biliary tract including the extrahepatic bile duct, cystic duct, and gallbladder at the insertion of laparoscope [6]. However, the time interval greatly depends on the individual liver function. Recently, Verbeek et al. [16] reported that a prolonged interval (24 h) permitted optimal near-infrared cholangiography with minimal liver background fluorescence. Therefore, we have to consider the ICG injection time (at the time of endotracheal intubation [12] or 10–15 min [8], 20 min [14], 30 min [10, 11], or 1 h [6, 11] before surgery according to the individual conditions).
Regarding the dose of ICG, we adjusted 2.5 mg/ml and administered 1 ml/body intravenously [6], and another institutions reported 0.1–0.5 mg/ml/kg or 2, 5, 10 ml/body of 2.5 mg/ml as a bolus in clinical use [6–19]. These dose differences are not greatly influenced in actual visualization during surgery. Furthermore, the duration of fluorescence (biliary secretion of ICG) was reported to last until 20 h after intravenous injection [19]. Therefore, there is no anxiousness about the duration of adequate fluorescence imaging during open or laparoscopic cholecystectomy.
Although the injection time and dosage of ICG are important factors to obtain a sufficient fluorescence imaging, the penetration depth of infrared light through tissue also relates to an identification of biliary tract. Mitsuhashi et al. [10] reported that the penetration depth was 3–5 mm and the intensity of fluorescence was affected to tissue thickness. Therefore, the thickness of the gallbladder wall or the volume of adjacent connective tissues is greatly affected by obesity or edema and dense adhesion due to acute or chronic inflammation during cholecystectomy. To resolve this issue, we introduced an originally manufactured transparent flat plastic device (TFPD), and its compression over the biliary tract led to increase the intensity of fluorescence and improved an identification of fluorescence imaging of the biliary tract [6]. It is a useful device to check the biliary anatomy and minimize the intraoperative complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree