Fig. 1
Schematic representation of the proposed mechanism underlying binding of nitroimidazole tracers in hypoxic cell. Reprinted with permission from Stroke 2008, 39, 1629–1637. Copyright (2008) American Stroke Association
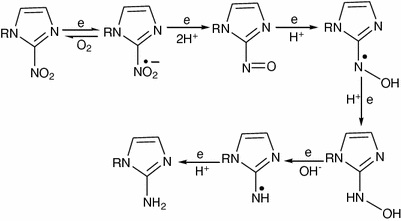
Fig. 2
Proposed mechanism for nitroimidazole reduction
In normal oxygen level, the free radical anion is rapidly reversed to its original compound (Fig. 2). The rate of oxidation is dependent on the intracellular concentration of oxygen. In hypoxic tissue, the reduced compound is not able to be reoxidized and is further reduced, resulting in association of the reduced nitroimidazole with various intracellular components. The association is not irreversible, since these agents clear from hypoxic tissue over time. So, under hypoxic conditions, bioreductive metabolism leads to further stepwise reduction to amine (6e−) derivatives (Fig. 2), and binds to cell components, thus the associated radiolabel is selectively retained in hypoxic cells. The cellular components that covalently bind the free radical-type nitroimidazoles have not been clearly identified, but might be intracellular macromolecules such as proteins or DNA (Nunn et al. 1995). Labeled nitroimidazole derivatives are therefore potential radiopharmaceuticals for imaging hypoxic areas. So, development of radiolabeled derivatives of nitroimidazole for hypoxia imaging is important for improving clinical outcome.
Naturally occurring nitroaromatic compounds are not common, but most classes of organisms including bacteria, protozoa, fungi, plants, and mammals can metabolize them using nitroreductase. It is difficult to say which particular enzyme is responsible for reduction of the nitro group in mammalian cells, but several enzymes are capable of this. Various nitroreductase enzymes are associated with the cytoplasm, microsomes, and mitochondria (McManus et al. 1982; Walton and Workman 1987).
Particularly for nitroimidazole derivatives, as discussed above, the amount of tracer delivered to the target via the blood–brain barrier (BBB) and faster washout from normoxic tissues with the high rate of trapping in hypoxic tissue is very important, as this determines the duration of the experiment sufficient to reach adequate contrast between normoxic and hypoxic tissue, as well as the presence of the intracellular macromolecular components necessary for binding (Nunn et al. 1995).
[18F]FMISO is the first and most widely studied nitroimidazole agent for in vivo PET imaging (Lee and Scott 2007; Martin et al. 1989; Rajendran et al. 2006). It has been evaluated extensively for detection of tumor hypoxia preclinically using different animal models. The first clinical study to image tumor hypoxia using [18F]FMISO was performed by Rasey et al. (1996), who used [18F]FMISO to quantify the hypoxic fraction in patients with lung, head and neck, and prostate cancers. Both animal and clinical studies using [18F]FMISO showed feasibility of this tracer as a hypoxia imaging agent (Kubota et al. 1999). However, [18F]FMISO has failed to obtain wider acceptance for routine clinical application because it is rather lipophilic, which may contribute to the finding that hypoxia-specific accumulation is rather slow. The need to wait several hours to permit clearance of the agent from the normoxic background tissue (contrast between lesion and background typically <2:1 at about 90 min after injection) is a serious fault of 18F-labeled agents having a relatively short half-life (110 min). In addition, due to the relatively low uptake of [18F]FMISO in hypoxic tissues, its clinical application has seen limited success. Therefore, considerable efforts have been made to develop more feasible compounds for clinical use, i.e., nitroimidazoles with either increased specific tumor uptake or faster clearance. Clinical experience with nitroimidazole agents other than [18F]FMISO is limited. However, one such candidate used for clinically is 18F-labeled fluoroerythronitroimidazole ([18F]FETNIM) (Yang et al. 1995; Gronroos et al. 2004), which is more hydrophilic than [18F]FMISO and shows rapid elimination from nontarget tissues via excretion through the urinary pathway. The log P value of [18F]FETNIM was reported to be 0.17.
[18F]FETNIM used in patients with head and neck cancer showed higher and heterogeneously distributed uptake in tumors than in adjacent neck muscle. Also, the tumor-to-blood ratio with [18F]FETNIM at 4 h after injection was significantly higher than with [18F]FMISO. Although it showed lower background than [18F]FMSIO at 90 min post injection, it was found to be largely perfusion dependent (Lehtio et al. 2001). Halogenated nitroimidazoles, such as 1-α-d-(2-deoxy-2-[18F]fluoroarabinofuranosyl)-2-nitroimidazole ([18F]FAZA) (Grosu et al. 2007; Piert et al. 2005; Postema et al. 2009; Souvatzoglou et al. 2007), 1-(5-[123I]iodo-5-deoxy-β-d-arabinofuranosyl)-2-nitroimidazole (iodoazomycin arabinoside) ([124I]IAZA) (Reischl et al. 2007), 2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3-[18F]pentafluoropropyl) acetamide ([18F]EF-5) (Dolbier et al. 2001; Komar et al. 2008; Yapp et al. 2007), and fluoroetanidazole ([18F]FETA) (Rasey et al. 1999), are other known hypoxia imaging agents (Fig. 3) developed to improve imaging performance by improving the target-to-nontarget ratio by increasing excretion rates. [18F]FAZA, developed to undergo more rapid clearance from blood and nontarget tissues than [18F]FMISO, was introduced to image tumor hypoxia with PET (Sorger et al. 2003). Because of its improved imaging properties, [18F]FAZA is recommended for further preclinical and clinical study for imaging tumor hypoxia. Compared with [18F]FMISO, [18F]FAZA displays a lower octanol:water partition coefficient (log P = 1.1) (Kumar et al. 1999), which indicates the potential for both rapid diffusion through tissue and faster renal excretion for [18F]FAZA (Kumar et al. 1999). Also, the significantly lower tumor-to-blood ratio resulting from [18F]FAZA is related to either renal or hepatobiliary excretion, leading to a lower radiation burden and more favorable imaging result than with [18F]FMISO (Sorger et al. 2003). Another agent is [18F]fluoroetanidazole ([18F]FETA), which is a well-known 2-nitroimidazole analog with P value of 0.16 (Barthel et al. 2004) and also showed significantly lower levels of retention in the liver and lung than those of [18F]FMISO (Rasey et al. 1999).
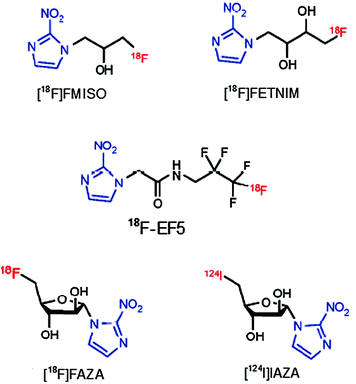

Fig. 3
Known nitroimidazole agents for hypoxia imaging
However, being more hydrophilic compounds, diffusion of PET tracers into tumoral tissues might be limited (Hoigebazar et al. 2011). So, a new class of more lipophilic derivatives, 2-(2-nitroimidazol-1-yl)-N-(3,3,3-trifluoropropyl)-acetamide (EF3) and EF5, were developed. Preliminary animal experiments involving these fluorinated derivatives showed more homogeneous distribution in normal tissues along with clearance through the kidneys and accumulation in hypoxic tumors (Busch et al. 2000; Koch 2002).
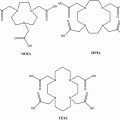
However, production of these radiotracers is limited on cyclotron systems, which are expensive as well as difficult to handle. Other than cyclotron-produced radioisotopes, an alternative method to label biomolecules is use of 68Ga, which can be obtained from a commercially available radionuclide generator system (Antunes et al. 2007; Breeman and Verbruggen 2007; Green and Welch 1989; Hnatowich 1977; Jeong et al. 2008; Shetty et al. 2010a, b). 68Ga is an economical alternative to cyclotron-produced radionuclides. Compounds characterized by high hydrophilicity were thought to be better for imaging hypoxia because of rapid blood clearance and high target-to-nontarget ratio. Recently, 68Ga-labeled nitroimidazole analogs were developed (Hoigebazar et al. 2010, 2011) for PET hypoxia imaging (Fig. 4). These derivatives showed elevated uptakes in hypoxic condition compared with normoxic condition in in vitro study. Also, good tumor uptake at 30 and 60 min and high tumor-to-muscle ratios were demonstrated by biodistribution and PET studies (Fig. 5).
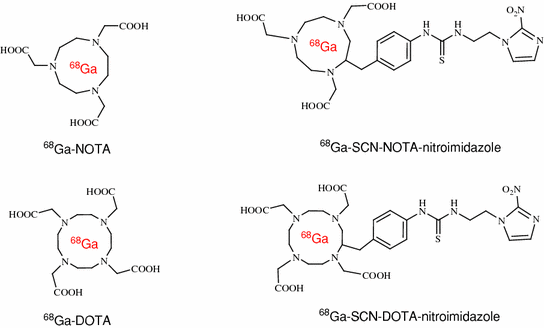
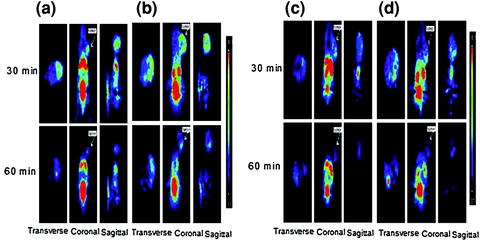

Fig. 4
Structures of 68Ga-labeled nitroimidazole derivatives

Fig. 5
Small-animal PET images of mice bearing a CT-26 xenograft on the right shoulder: a 68Ga-NOTA-nitroimidazole (26.64 MBq/0.15 mL), b 68Ga-DOTA-nitroimidazole (24.05 MBq/0.15 mL), c 68Ga-SCN-NOTA-nitroimidazole (34.04 MBq/0.15 mL), and d 68Ga-SCN-DOTA-nitroimidazole (39.26 MBq/0.15 mL) injected through tail vein at 30 and 60 min post administration. Reprinted with permission from Hoigebazar et al. (2010). Copyright (2011) American Chemical Society. Reprinted with permission from Hoigebazar et al. (2011). Copyright (2011) Elsevier
6 Copper(II)-Diacetyl-bis(N 4-methylthiosemicarbazone) (Cu-ATSM) as Hypoxic Agent
Other than nitroimidazole agents, 64Cu-ATSM has been developed for hypoxia PET (Fujibayashi et al. 1997). 64Cu-ATSM is an agent that shows rapid delineation of tumor hypoxia (<1 h) with high tumor-to-blood ratios ≫2.0. It has been shown to be selective for hypoxic cancers and ischemic myocardial tissue. Hypoxia selectivity of 64Cu-ATSM was reported first in an isolated rat heart ischemia model (Fujibayashi et al. 1997).
In the chemistry of copper, there are two principle oxidation states, I and II, whose biochemistry and metabolism are known in human as they are omnipresent. Use of copper-labeled radiopharmaceuticals for PET is attractive because of the increasing availability of four positron-emitting radionuclides of copper: 60Cu (t 1/2 = 0.40 h, β+ = 93%, EC = 7%), 61Cu (t 1/2 = 3.32 h, β+ = 62%, EC = 38%), 62Cu (t 1/2 = 0.16 h, β+ = 98%, EC = 2%), and 64Cu (t 1/2 = 12.7 h, β+= 17.4%, EC = 43%) (Blower et al. 1996). 62Cu can be produced by a generator (62Zn/62Cu generator) system (Fukumura et al. 2006; Haynes et al. 2000), whereas 60Cu, 61Cu, and 64Cu are produced by cyclotron (McCarthy et al. 1997, 1999) using reliable and reproducible target methods. Among copper isotopes, 64Cu is most commonly used, as it is well suited for PET studies with half-life of 12.7 h and β+ maximum energy of 0.66 MeV. This long half-life allows distribution of the nuclide from regional production centers to imaging centers that do not have an in-house cyclotron. 64Cu also decays by electron capture (43%) and β− (43%) and therefore has been studied as both a diagnostic and therapeutic radionuclide (Blower et al. 1996).
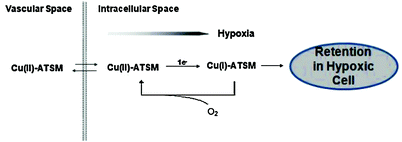

Fig. 6
Schematic representation of the proposed mechanism for Cu-ATSM in hypoxic cell
A comparative biodistribution study was carried out in BALB/c mice bearing EMT6 tumors after intravenous injection of 64Cu-ATSM and copper-pyruvaldehyde-bis(N 4-methylthiosemicarbazone) (64Cu-PTSM) (Lewis et al. 1999). The data showed optimal tumor uptake of both agents at 10 min post injection, suggesting a rapid trapping mechanism for these agents in solid tumors. Ex vivo autoradiography of tumor slices after co-injection of 64Cu-ATSM and 60Cu-PTSM into the same animal was also studied. The result obtained showed uniform spreading of 60Cu-PTSM throughout the EMT6 tumor, but heterogeneous uptake of 64Cu-ATSM suggested trapping of this agent in hypoxic region of the tumors.
A proposed mechanism of Cu-ATSM retention was first reported by Fujibayashi et al. According to their research, Cu(II)-ATSM reduction only occurred in hypoxic cells and it was then irreversibly trapped (Fujibayashi et al. 1997) (Fig. 6). Later, a mechanistic study of Cu-ATSM in tumor cells was performed and found that Cu-ATSM was reduced mainly in microsome/cytosol fraction rather than in mitochondria (Obata et al. 2001). The reduction process in the microsome/cytosol (heat-sensitive) was enhanced by adding exogenous NADPH, indicating enzymatic reduction of Cu-ATSM in tumor cells. Additionally, it was found that the bioreductive enzymes, NADH-cytochrome b5 reductase and NADPH-cytochrome P450 reductase in the microsomes, play a major role in the reductive retention of Cu-ATSM in tumors, and this enzymatic reduction was enhanced by induction of hypoxia. Further studies of Dearling et al. (2002) and Maurer et al. (2002) suggested that Cu(II)-ATSM undergoes reduction in both normoxic and hypoxic cells, resulting in unstable Cu(I)-ATSM. This unstable species would be reoxidized to Cu(II)-ATSM in normoxic cells and diffuse back out of the cells. However, in case of hypoxic cells, it would completely dissociate and be irreversibly trapped inside the cells.
The crystal structure of Cu(II)-ATSM could be determined, but isolation of Cu(I) was not possible (Cowley et al. 2002). However, the novel dimeric species [Cu2(ATSMH2)2]2+ could be purified as a [PF6]− salt. The X-ray crystal structure of this agent showed a dimeric structure with each of the ATSM ligands acting as a bidentate N–S donor to each Cu(I) ion to generate a novel helical structure which is unprecedented for bis(thiosemicarbazone) complexes. Cu-ATSM has several advantages over other radiopharmaceuticals used for hypoxia PET, including a simple synthesis method, faster clearance from normoxic tissue allowing a short waiting time for imaging, and a simpler method for quantification. 64Cu-ATSM (Lewis et al. 1999; Vavere and Lewis 2007) and 60Cu-ATSM (Lewis et al. 2008) show fast clearance rate from normoxic tissue and allow a shorter waiting time for imaging. Although Cu-ATSM is not perfect and may not be applicable for all cancer types, it holds exceptional potential as a hypoxia PET agent.
7 Retention of Radiolabeled Compounds in EMT6 Cells
Lewis et al. performed a washout study using Cu-ATSM in which the radioactivities from normoxic and hypoxic cells were observed. Samples were taken at 1, 5, 15, 30, 45, and 60 min after addition of radiotracer. Over 42% of 64Cu-ATSM was washed out from normoxic cells after 1 h, but in hypoxic cells this value was only 27%, suggesting selective retention of the complex in a more reducing cellular environment (Lewis et al. 1999). The hypoxic retention of 64Cu-ATSM was proved to be a reversible phenomenon dependent only pO2 but not due to irreversible cellular damage such as membrane disruption (Fujibayashi et al. 1997). 64Cu-ATSM is a small lipophilic molecule having neutral square planar structure with high membrane permeability and low redox potential for selective retention within hypoxic tissue (Green 1987; Taniuchi et al. 1997). BALB/c mice were injected subcutaneously into each flank with EMT6 cells from cell culture. After 10 days, 64Cu-ATSM (0.2 MBq) was injected through the tail vein and biodistribution studies were carried out. The uptake of 64Cu-ATSM into the EMT6 tumor showed optimal uptake after 10 min.
Direct comparison of cellular uptake of 64Cu-ATSM and [18F]FMISO in vitro was done by Lewis et al. They found selective uptake of both tracers in hypoxic cells. However, the peak cellular uptake of [18F]FMISO was approximately 15% that of 64Cu-ATSM and occurred after a much longer incubation time (2 h versus 15 min) (Lewis et al. 1999, 2001). This reflects the greater membrane permeability of 64Cu-ATSM than of [18F]FMISO. Also, these studies were confirmed in an in vivo rodent tumor model, in which uptake of 64Cu-ATSM was shown to be dependent upon tissue pO2, with greater retention of this tracer in hypoxic tissues. These preclinical studies suggested that 60Cu-ATSM could be used clinically to identify hypoxic components of human tumors by PET. However, production of 60Cu and 64Cu requires a special solid target processing system and expensive target material, and hence, its use is likely to be limited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree