Fig. 4.1
Standard, five-session read design for oncology trials
Reader Allocation for Oncology Trials
The generally accepted industry standard reader allocation for a pivotal Phase 3 oncology trial in which progression or response as captured objectively in an imaging read is a primary or secondary endpoint is to employ two radiologists as primary readers who assess 100 % of the data. In the event that there is disagreement on the main points of assessment, a third reader reviews the primary readers’ responses and selects the assessment with which he/she is in greatest agreement. This is referred to as the 2 + 1 reader allocation. Alternative read paradigms are presented in the read methodologies chapter (Chap. 5).
Purpose of the Global Session
An additional consideration regarding an oncology read design and reader model includes whether to include a session to enable the readers to review all of the images from baseline to the final time point at one time and to allow the reader to change previous assessments. This is known as a global session. Although the selection of target and nontarget lesions at baseline cannot be altered, this session allows the reader to modify a response assessment at a subsequent time point.
In the global session, the reader has the opportunity to review such critical progression- and response-related assessments, such as whether a lesion identified at a specific time point as a new lesion was in fact simply a benign lesion which may have “appeared” as new based on the phase of contrast (i.e., a hepatic hemangioma). Additionally, an evaluation of “unequivocal progression of nontarget disease” at a specific time point that ultimately is not consistent with target tumor burden and was likely a false-positive can also be noted in this session.
While the global session allows the reader to review and reevaluate key assessment criteria, identify changes, and provide the rationale for such modifications, both the original assessment and subsequent reassessment are captured in the read database and become permanent entries in the audit trail. However, if an original assessment is changed in the global session, only the final assessment is used to calculate overall progression or response.
The intent of implementing this session is to enable the readers to provide a more accurate assessment based on the overall burden of disease and the evolution of their radiographic findings over time. This affords the readers an opportunity to use their best clinical judgement during the independent read and more closely mirrors the clinical practice setting.
The Eligibility Read
Another type of read that pharmaceutical companies consider when planning a read design is the eligibility assessment. The eligibility read typically captures a subset of data and provides a high-level assessment of that data to determine whether potential subjects meet the inclusion criteria for a study. Sponsors of a clinical trial often employ an eligibility read if the personnel at the sites do not have as robust an understanding of the “measurability” of disease as the central readers or a sophisticated quantitative measurement has to be used, such as hippocampal volume in Alzheimer’s disease. Sponsors also implement an eligibility read to mitigate the effect of PIs who may be overly eager to push patients with late-stage disease into a clinical trial.
There are several different approaches to eligibility reads, but the two designs illustrated in Figs. 4.2 and 4.3 are the most prevalent. For both scenarios, it is important to establish in the Imaging Charter how the information is going to be conveyed to the sites and for the site PIs to understand that the assessment by the central readers takes precedence over assessments made at the site. In addition, the sponsor of a study must work closely with the imaging core lab to ensure that the data can be transferred quickly and that a timely assessment can be obtained and relayed back to the sites prior to patient randomization.
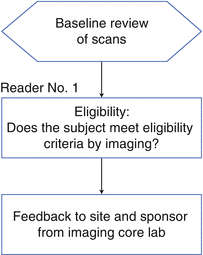
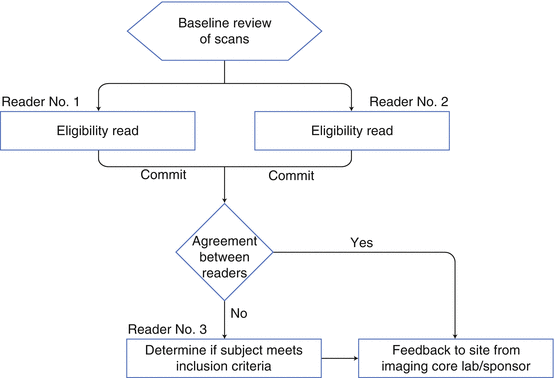

Fig. 4.2
Standard eligibility read design for oncology trials

Fig. 4.3
Alternative eligibility read design for oncology trials
Due to the rapid turnaround required for eligibility reads, usually a pool of readers has to be selected. The challenge is then to ensure all the readers evaluate images in the same manner or are “calibrated” to each other. A well-designed calibration program has to be developed, deployed, and continued throughout the course of the study.
Reading for Confirmation of PD in Oncology
In addition to these scenarios, sponsors sometimes request that the imaging core lab confirm progressive disease (PD) as part of the independent read (Fig. 4.4). This additional step is implemented when there is high risk of the sites declaring progressive disease prematurely, which often results in the patient being moved into another treatment arm of the clinical trial. When this happens, the patient’s data set is “censored” by the sponsor and remains incomplete. When there is disagreement between the site read and the central read, the central read is considered to be truth. If the imaging core lab determines that PD has not been reached, that subject remains in the study and increases the likelihood that the data set will be complete.
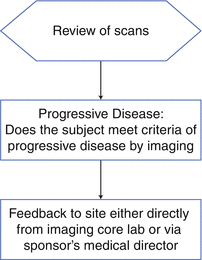

Fig. 4.4
Read design for confirmation of PD in oncology trials
Selecting and Screening Readers
Typically, medical and/or scientific staff at the imaging core lab with support from a clinical-operations type of department screens all potential readers to evaluate their past clinical trial experience. A reader’s qualification depends upon their expertise and the type of study. The Imaging Charter for a particular trial will help the imaging core lab to understand what is expected of each reader. Reader qualification is based on formal training, performance on previous trials, experience with disease indication, image modality, and the reader’s ability to meet timelines. All readers should be board certified (or international equivalent) and, depending on the indication, may need to have additional qualifications. Imaging core labs will recommend that readers have a background check to ensure that the FDA has not debarred them from participating in clinical studies. Infrequently, sponsors may request the reader to complete a Financial Disclosure Form or a 1572.
A sound selection and screening model will identify readers who are considered experts in different therapeutic areas and/or modalities who are working in centers of excellence. These are often key opinion leaders who are eager to work in translational medicine, contributing to the clinical care of their patients as well as participating in clinical research.
Additionally, the medical and/or scientific staff at the imaging core lab should consider each project individually and work with a team to cross-reference a database of existing experts or to identify new readers, if needed.
After identifying potential readers, the imaging core lab contacts the reader and describes the nonconfidential details of the study as well as the projected time commitment. If the reader is able to commit to the study, their CV and reader rate is provided to the sponsor. If the study sponsor and reader are in alignment, the imaging core lab will execute a reader agreement and oversee the training, monitoring, and completion of the reader’s participation in the trial. Study sponsors should approve all readers before they are assigned to a project.
Reader Training/Mock Read
Imaging core labs will provide training for the readers on a per-study basis. The selected readers often travel to the imaging core lab for a “Mock” read and are introduced at this time to the read system the imaging core lab has developed and to the read methodology. Training cases are evaluated during the session, both in a group setting if there is more than one reader, and individually by each reader, allowing the readers to come together at the end of the session to discuss methodology and rules for the reader. The imaging core lab provides documented read rules based on the read design outlined in the Imaging Charter, as well as the salient points discussed at the Mock read. The readers are then to follow the read rules to safeguard that a reader is consistent throughout the length of the study as well as among multiple readers.
Reader Monitoring
Imaging core labs often perform an evaluation of read results to ensure the adjudication rate (the percentage of cases between primary readers who disagree on an assessment and subsequently require the adjudicator to select one of the assessments) does not exceed the threshold that the study sponsor and the imaging core lab established. This assessment is also known as inter-reader variability analysis.
The same sort of analysis is often done to compare a reader’s interpretation to their own earlier assessments of a selected set of cases. This intra-reader variability evaluation is performed to confirm that a given reader’s rate of agreement with their earlier assessments demonstrates internal consistency in applying the response criteria and reader rules. This rate should similarly be monitored on an ongoing basis to ensure it does not exceed the threshold established by the study sponsor and the imaging core lab. The frequency of such evaluations should be determined at the inception of the read-monitoring plan.
Reader monitoring also safeguards that the readers are adhering to the reader rules and the criteria of the read. If the imaging core lab offers reader monitoring, the evaluation should be conducted on a regular basis and per the read design outlined in the Imaging Charter.
The methodology of reader monitoring usually includes an early assessment after a predetermined number of cases have been read by the primary readers and analyzed by the adjudicator, if necessary. The data are assessed based on the points of adjudication identified by the sponsor and the imaging core lab. Multiple statistical methods are applied as part of the reader-monitoring process. One example, the Bland-Altman plot, can be seen in Fig. 4.5.