(1)
Department of Biomedical Imaging and Image-guided Therapy, Vienna General Hospital, Medical University of Vienna, Währingergürtel 18-22, 1090 Vienna, Austria
(2)
Department of Emergency Radiology, Cardarelli Hospital, Naples, Italy
(3)
Academic Health, Rietgrabenstrasse 76b, CH-8152 Opfikon, Switzerland
1 Community-Acquired Pneumonia
1.1 Introduction
Community-acquired pneumonia (CAP) is a frequent lower respiratory tract infection.
It is highly influenced by the geographic area and the population.
The microorganisms may reach the lower respiratory tract from inhaled air or from infected oropharyngeal secretions (Vincent et al. 1995). Droplet transmission from human to human is another usual mode of spread of the pulmonary infection, especially in immunocompetent patients and young children.
Clearance by mucociliary system can filter infective organisms 5–10 nm in diameter, so that microorganisms between 1 and 2 nm can reach the alveolar tissue. The alveolar infection depends on the balance between the virulence of the microorganism and body defenses due to cellular phagocytosis. There are several factors predisposing to the development of CAP as cardiovascular diseases, diabetes, uremia, immunodeficiency, obstructive pulmonary chronic disorders with mucociliary clearance insufficiency, and age.
Alcoholic patients and people with poor oral hygiene are particularly sensitive to develop pulmonary infections (Barlettand Finegold 1974).
The clinical presentation of pneumonia is frequently represented with sudden symptoms as high fever, cough, purulent expectoration, and deep chest pain. Neutrophilia is common.
CAP is classified into three main groups: lobar pneumonia, bronchopneumonia, and interstitial pneumonia (Bhalla and Mc Loud 1998).
Lobar pneumonia appears in the lung parenchyma periphery and then diffuses up to the hilum. It is generally limited to one pulmonary segment or lobe.
Bronchopneumonia occurs when infectious microorganisms produce acute bronchial mucosal inflammation and spread through the airway into the alveolar spaces determining pulmonary consolidation.
Interstitial pneumonia is determined by the infection of pulmonary interstitium and is mainly caused by viral organism.
The same organism may produce several different patterns that depend on the balance between the microorganism charge and the body immunity defenses.
The spectrum of bacteria responsible for CAP includes Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, and Klebsiella pneumoniae (American Thoracic Society 1995; Marom et al. 1999; De Paso 1991).
Among the types of CAP, S. pneumoniae is the most frequent causing bacteria, and it is associated with a high mortality rate in the elderly and childhood.
Haemophilus influenzae also is one of the most frequent bacteria causing CAP, because it frequently colonizes the human upper respiratory tract, especially the nasopharynx, and is considered to form part of the normal respiratory flora. It is also an important cause in the acute exacerbation of chronic obstructive pulmonary infectious disease (Kofteridis et al. 2009).
The viruses that most commonly cause pneumonia are respiratory syncytial virus, herpes simplex, parainfluenza virus, influenza virus, adenovirus, and cytomegalovirus.
The funguses that most commonly cause pneumonia are Pneumocystis, Aspergillus, and Candida.
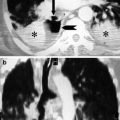
Pulmonary emphysema and bronchiectasis are frequently seen in patients with CAP because chronic obstructive pulmonary disease is a predisposing factor for developing pulmonary infection (Reittner et al. 2000a) (Fig. 1).
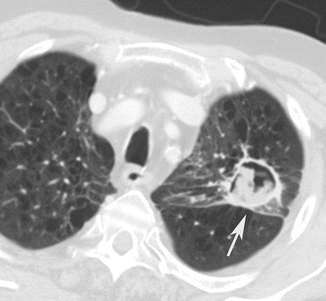

Fig. 1
Fungal abscess (white arrow) in diffuse pulmonary emphysema. Post-contrast CT axial scan demonstrates large air-containing dishomogeneous focal area, with peripheral-enhanced irregular wall, characteristic of fungal abscess, with underlying pulmonary emphysema
1.2 Lobar Pneumonia
Depending on the severity of the pulmonary alveolar tissue involvement, the pattern of the infection may be patchy or homogeneous. The consolidation is usually confined to one lobe, although multilobar involvement is not uncommon (Fig. 2). Because the bronchial tree is not involved, there is no volume loss of the inflamed pulmonary segment or lobe.
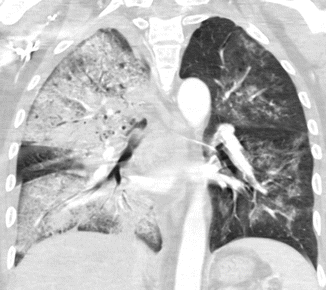

Fig. 2
Bilateral pneumonia. CT coronal scan demonstrates lobar consolidation of right upper and lower lobes with no volume loss and bulging of interlobar fissure. On the other side, there are lobular areas of consolidation. Air bronchograms within the consolidation areas are preserved
Lobar pneumonia is characterized by the filling of alveolar spaces by edema full of white and inflammatory cells. The inflammation generally begins at the lung mantle and then diffuses to the entire lobe through Kohn pores and peripheral small airways; usually it is limited by an interlobar fissure. The air bronchograms within the consolidation area are preserved (Fig. 2). Nodular pneumonia is frequently caused by fungal infections and is prevalent in young or immunocompromised patients.
The pneumonia could be complicated with cavitation and abscess (Fig. 3), necrosis of the inflamed lung parenchyma, and loculated empyema (Muller et al. 2007).
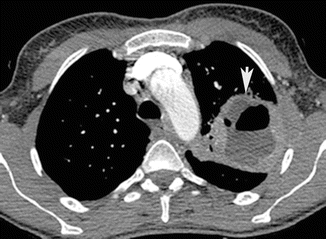

Fig. 3
Lung abscess. Post-contrast CT axial scan demonstrates large focal area of decreased attenuation with air-fluid level, with rim enhancement, characteristic of lung abscess (arrow) of the left upper lobe
Abscesses develop in up to 30 % of cases, are usually solitary, and typically have an irregular wall. They could rupture into the pleural space with the development of pneumothorax and empyemas (Fig. 4). Abscesses may also erode into bronchial tree and produce air-containing cavities with fluid levels. The consequent bronchial aspiration and diffusion of infection can determine patchy consolidation or nodules in dependent portions of both the lungs (Fig. 5). The consolidations are usually multilobar and bilateral in distribution.
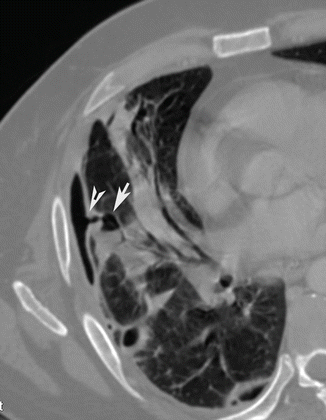
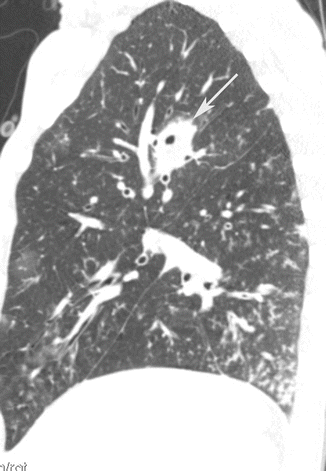

Fig. 4
Post-contrast CT axial scan demonstrates a small air-containing pulmonary cavity with fluid level (arrow) ruptured into the pleural space (arrow tip) with a small left pneumothorax

Fig. 5
CT sagittal scan demonstrates multiple nodules in dependent portions of the lung
A large solitary abscess or primary lung abscess can develop in a patient without underlying lung inflammation as a consequence of aspiration of oropharyngeal secretions in combination with impairment of systemic defense condition.
Necrotizing pneumonia, generally determined by Gram-negative bacteria, produces exfoliated pulmonary tissue within a cavity or diffuses microabscesses secondary to thrombosis of the vessel that supply the consolidation. Sometimes necrotizing pneumonia consists of a fulminant process associated with focal areas of necrosis that results in abscesses that can coalescence, resulting in large cavities that may exhibit thick fibrotic walls if they are chronic. There are large cavities with concomitant small cavities frequently associated with empyema (Winer-Muram et al. 1993). This feature is frequent in immunocompromised patients.
1.3 Bronchopneumonia
Bronchopneumonia or lobular pneumonia is characterized by a peribronchiolar inflammation with thickening of peripheral bronchial wall, the diffusion of inflammation to the centrilobular alveolar spaces, and development of nodules (Fig. 6). From the centrilobular tissue, the inflammation can spread to lobular (Fig. 7) or subsegmental areas giving a consolidation. The areas of consolidation may be patchy or confluent, multilobar, unilateral, or bilateral (Fig. 8). Because the process involves the airways, it can determine a loss of volume of the affected pulmonary segment (Ito et al. 2009).
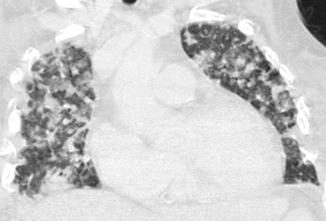
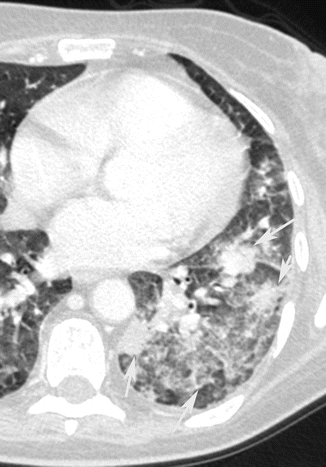
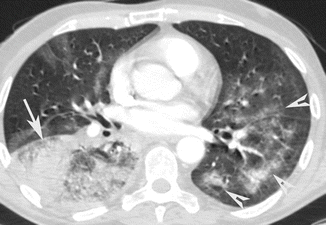
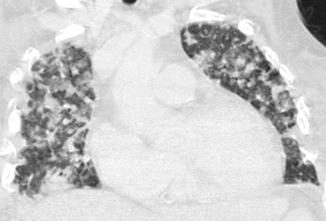
Fig. 6
Bronchopneumonia. CT coronal scan demonstrates multiple, bilateral, centrilobular nodules

Fig. 7
Bronchopneumonia. CT axial scan demonstrates multiple, lobular (arrow), patchy, confluent consolidation areas of the left lower lobe

Fig. 8
Bronchopneumonia. CT axial scan demonstrates multiple confluent areas of consolidation (arrow) of the right lower lobe and patchy lobular areas of consolidation of the left lower lobe (arrow tips)
1.4 Interstitial Pneumonia
Interstitial pneumonia is frequently caused by virus.
The viral pneumonia begins with the destruction and exfoliation of the respiratory ciliated and mucous cells. The interstitial septa and the bronchial and bronchiolar walls become thickened for the inflammation process and lymphocyte interstitial infiltrates (Fig. 9). The consequent interstitial pneumonia has often a patchy appearance, frequently with coalescence, involving predominantly the peribronchial portions of the pulmonary lobules (Shiley et al. 2010). With the progression of inflammation, the alveolar sacs fill with exudate that could be hemorrhagic. As consequence a hyaline membrane can develop in alveolar spaces.
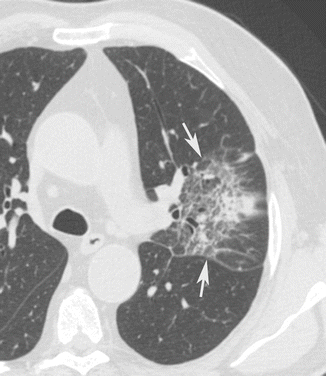

Fig. 9
Interstitial pneumonia. CT axial scan evidences that the lingular interstitial septa, bronchial and bronchiolar walls are thickened for an inflammation process (arrows). There is also a central lobular consolidation with patchy appearance
The pneumonia can heal completely, but sometimes a chronic interstitial fibrosis can occur.
1.5 Radiographic Features
Chest radiography represents an important initial examination in all patients suspected of having pulmonary infection and for monitoring response to therapy.
Its role is to identify the pulmonary opacities (Fig. 10), their internal characteristics and distribution, pleural effusion, and presence of other complications as abscesses and pneumothorax.
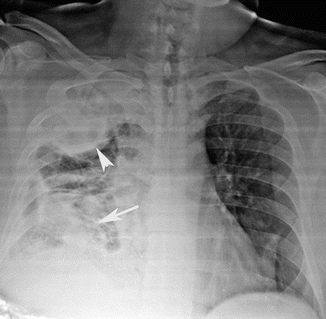

Fig. 10
Chest radiograph shows a right upper lobe opacity (arrow tip) and a nonhomogeneous consolidation of the right lower lobe (arrow)
Radiographic manifestations depend on the immune status of the patient and the presence of preexisting lung chronic alterations as emphysema, chronic bronchitis, and bronchiectasis and vary somewhat among the various species of causing microorganisms.
The most common radiographic findings include hazy ground-glass opacities, confluent or patchy airspace consolidations, cavitations, and poorly defined linear or reticular-nodular opacities (Chastre et al. 1998).
These alterations may involve mainly the perihilar regions, lower lung zones, or upper lung zones. They are usually unilateral but may involve both lungs. Other findings are pleural effusion and hilar adenopathy.
In some cases, the radiographic findings are suggestive or consistent with the diagnosis of pneumonia and are sufficiently specific in proper clinical context to preclude the need for additional imaging (Fig. 11) (Franquet 2001).
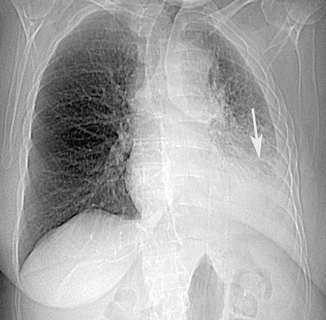

Fig. 11
Posteroanterior chest radiograph shows dense left lower lobe airspace consolidation (arrow) with pleural fluid
However, the bedside plain film, the projection effects, and the poor density resolution frequently limit the value of radiography as a diagnostic tool. Radiographic features are sometimes nonspecific, and the relatively low diagnostic accuracy of chest radiography can be improved with CT.
1.6 Computed Tomography Findings
CT has been shown to be more sensitive than x-ray plain film and provide additional information that affects diagnosis or management in up to 70 % critically ill patients (Heussel et al. 1997).
High resolution CT allows accurate assessment of airspace inflammation (Okada et al. 2012).
The distribution of parenchymal alterations is based on the evidence of their localization at CT scan. If the primary opacity is predominantly located in the inner third of the lungs, the pathology is classified as having a central distribution. A peripheral opacity is defined as a lesion localized in the outer third of the lungs. Zonal predominance is classified as either upper or lower. In the upper lung zone predominance, the alteration is located above the level of the tracheal carina, whereas in the lower zone predominance, it is located below the upper zone.
The CT findings include nodules, interlobular septal thickening, intralobular reticular opacities, ground-glass opacities, tree-in-bud pattern, lobar-segmental consolidation, lobular consolidation, abscesses, pneumatocele, pleural effusion, pericardial effusion, mediastinal and hilar lymphadenopathies, airway dilatation, and emphysema.
Nodules measure from 3 to 10 mm and are divided into three types: centrilobular nodules that are small nodules in centrilobular location, peribronchovascular nodules that are relatively larger nodules associated with the peribronchovascular bundle, and “random nodules” that are not associated with centrilobular structures or bronchovascular bundle.
The centrilobular nodules reflect a peribronchial inflammation with a centrilobular distribution (Fig. 12).
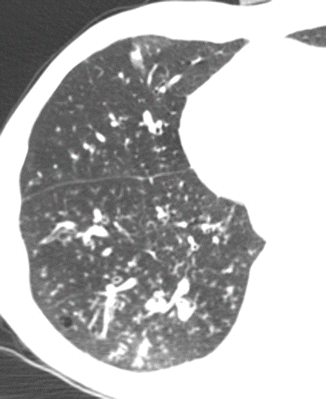

Fig. 12
CT axial scan evidences multiple centrilobular nodules and peribronchial inflammation with centrilobular distribution
They are defined as a dot-like opacities localized inside the center of a secondary pulmonary lobule. They are present around the peripheral pulmonary arterial branches or 3–5 mm from the pleura, interlobular septa, or pulmonary veins.
Nodules that are present in the bronchovascular bundle are called peribronchovascular nodules (Fig. 13).
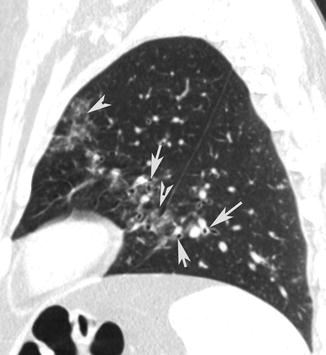

Fig. 13
Peribronchovascular nodules. CT sagittal scan shows small nodules (arrow tips) in the bronchovascular bundle and peribronchial inflammation (arrows)
Hemorrhagic pulmonary nodules have a central area of soft tissue attenuation surrounded by a halo of ground-glass attenuation (Primack et al. 1994).
Pulmonary hemorrhage in association with nodules occurs in patients with herpes simplex virus, candidiasis, and cytomegalovirus pneumonia (Thurlbeck et al. 1991)
Interlobular septal thickening are defined as abnormal widening of the interlobular septa. Intralobular reticular opacity is considered to be present when interlacing line shadows are separated by a few millimeters (Fig. 9) (Austin et al. 1996).
It is frequently associated with bronchial wall thickening. Thickening of the bronchovascular structures is defined as an apparent thickening of the bronchovascular bundle in comparison with the unaffected lung parenchyma (Fig. 14).
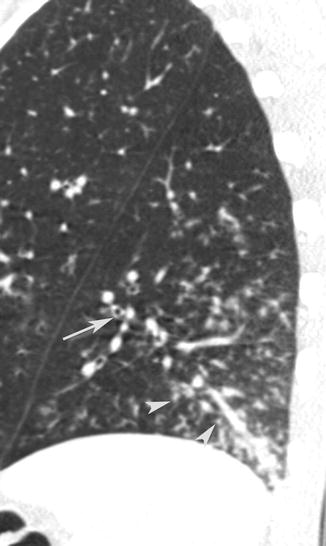

Fig. 14
CT sagittal scan shows small nodules (arrow tips) associated with thickening of the bronchovascular structures (arrow)
Ground-glass opacity was defined as hazy increased opacity with preservation of bronchial and vascular markings (Fig. 15). It is present particularly in pneumocystis and cytomegalovirus infections (Fig. 16).
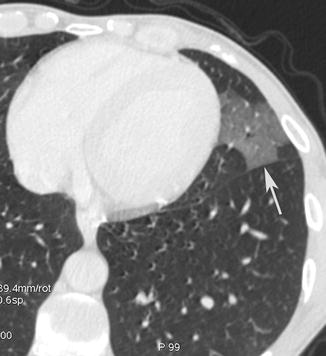
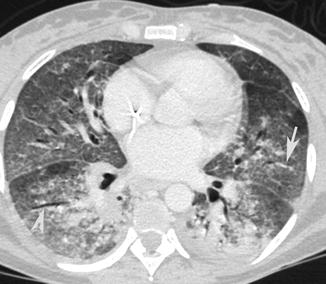

Fig. 15
Ground-glass opacity. CT axial scan evidences a lingular ground-glass opacity (arrow) with preservation of bronchial and vascular markings

Fig. 16
CT axial scan demonstrates multiple, confluent, bilateral ground-glass opacities with preservation of bronchial (arrow tip) and vascular (arrow) markings
Tree-in-bud pattern may be seen in a variety of bacterial, mycobacterial, fungal, and viral infections and consists of centrilobular branching tubular structures, bronchovascular bundle thickening, and nodules and reflects the presence of bronchiolar inflammation and filling of the bronchiolar lumen by inflammatory exudate (Fig. 17) (Aquino et al. 1996).
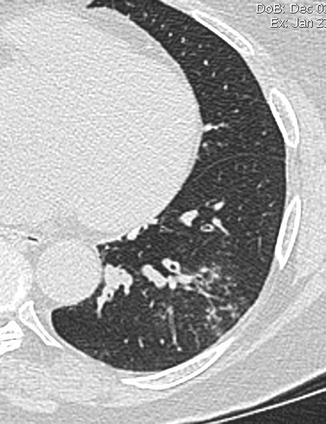

Fig. 17
Tree in bud. CT axial scan demonstrates centrilobular branching tubular structures, bronchovascular bundle thickening, nodules that reflect the presence of bronchiolar inflammation, and filling of the bronchiolar lumen by inflammatory exudate
Airspace consolidation is considered to be present when homogeneous increases in pulmonary parenchymal attenuation obscured the margins of vessels and airway walls (Fig. 2) (Hansell et al. 2008).
The CT findings in viral pneumonia are based on the evidence of small patchy opacities that can coalescence (Fig. 18), composed of multiple, poorly defined 2–3 mm nodules; multilobar involvement is frequent. Bronchial wall and peribronchial thickening frequently spread outward from the hila. Hilar adenopathy and pleural effusion are common.
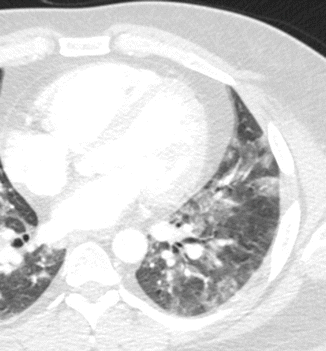

Fig. 18
Viral pneumonia CT axial scan evidences small patchy opacities of the left lower lobe
The abscess can drain into a bronchus that leads to the classic air-fluid level, present in up to 72 % of cases (Fig. 3). The complete drainage of the cavity can lead to a pneumatocele.
Pneumatocele is a frequent consequence of an abscess drained in the lumen of a bronchus; it is a gas-filled area with a thin wall that may increase and can get ruptured into the pleural space, causing a pneumothorax. It can also heal completely, fading from the periphery to form a tiny thin-walled air-cyst or a linear scar.
Pleural effusion is better detected with CT than with radiography and is often associated with a high risk of empyema that requires prompt early drainage.
Purulent pericarditis is a relatively rare and a rapidly fatal complication of pneumonia. The consequent development of cardiac tamponade may be rapid. At the beginning, heart size may be normal at chest radiograph, whereas CT can demonstrate early the pericardial effusion that requires prompt drainage for avoiding cardiac compression (Fig. 19) (Naidich et al. 1983).
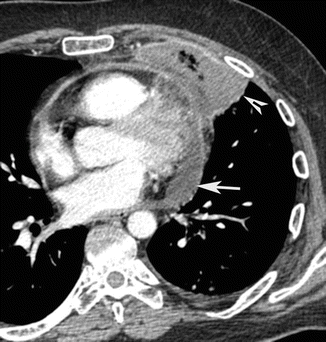

Fig. 19
Pulmonary abscess associated with purulent pericarditis. Post-contrast CT axial scan demonstrates a pre-pericardial lung abscess (arrow tip) of the left lower lobe associated with thickening of the pericardium and pericardial effusion (arrow)
Mediastinal and hilar lymphadenopathies are evaluated when the minimal diameter of a lymph node is larger than 10 mm. Lymph node enlargement is very frequent in pneumonia and generally involves the paratracheal, tracheobronchial, and subcarinal mediastinal chains.
Airway dilatation predisposes to pneumonia and is considered to be present if the bronchial size exceeded that of the near pulmonary artery.
Pulmonary emphysema is frequently associated with pneumonia and is defined as scattered or diffuse areas of low attenuation without internal vascular structures in comparison with normal pulmonary parenchyma (Fig. 1).
1.7 Conclusion
Pneumonia is the first leading cause of death due to infection worldwide (Vilar et al. 2004a).
Many Gram-positive, Gram-negative bacteria, funguses, and viruses can cause the infectious pulmonary disease, and the severity of pneumonia depends on the balance between the microorganism charge, the body immunity defenses, and the quality of the underlying pulmonary tissue.
Among the types of CAP, S. pneumoniae is the most frequent causing bacteria, has a tendency to develop antibiotic resistance, and is associated with a high mortality rate in the elderly and childhood (Musher et al. 2002).
Radiographically pneumonia is divided into three patterns: lobar pneumonia, bronchopneumonia, and interstitial pneumonia (Muller et al. 2007).
Chest x-ray film is routinely performed for the initial evaluation of patients with suspect pulmonary infection, and it is considered the recommended tool for the diagnosis of CAP. However, chest x-ray film not always evidences all the findings of pneumonia, and it is limited by the interobserver variability in interpretation.
Especially in not clear or even negative chest radiography pattern, in patients with clinical symptoms, CT is recommended, for its high spatial resolution and the ability to evidence very early and minimal pulmonary alterations indicative of pneumonia.
2 Atypical Infections
2.1 Definition
Community-acquired pneumonias have been traditionally divided into “typical” and “atypical” pneumonias. The main aim of such a classification was to help the clinician narrow the differential diagnosis and choose an appropriate therapy based on the likely offending organism (Murray and Mason 2010).
Typical pneumonia is characterized by infection with bacteria such as Streptococcus pneumoniae, Klebsiella pneumoniae, and Haemophilus influenzae. It presents with symptoms such as acute onset of high grade fever with chills, cough with purulent expectoration, and pleuritic chest pain. Further evaluation of these patients reveals an elevated white blood cell count, elevated CRP, and lobar or segmental consolidation on imaging studies (Murray and Mason 2010).
Atypical pneumonia, as the name explains, does not show these typical features. An early use of this term in the literature dates back to 1938 when cases of patients who presented with milder symptoms and protracted course came to notice (Reimann 1938). These patients had more generalized symptoms like malaise, body pains, and low-grade fever with dry cough and flu-like symptoms. The causative organism could not be cultivated on regular media or stained with regular staining methods. Mycoplasma was first isolated in 1944 and was attributed as the etiology of primary atypical pneumonia (Eaton et al. 1944). However, several other organisms cause atypical symptoms.
Although originally used to signify any infection that was unusual in presentation, atypical pneumonia is now a broad term that includes infections caused by common organisms like Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila and viruses (Marrie et al. 1981). Atypical, however, does not mean that they are rare; they account for almost 15–20 % of the cases of CAP (Arnold et al. 2007). Mycoplasma pneumoniae is the most common cause of atypical pneumonia followed by Chlamydia pneumoniae and Legionella pneumophila (Arnold et al. 2007). Apart from these conventional pathogens, there are emerging infections with newer organisms like Hantavirus, human metapneumovirus that can also cause atypical pneumonia. In view of the current radiological discussion and similar radiological appearances shared by mycoplasma and various viruses, we will also be discussing some additional infections in this chapter, which, although not considered routinely under the heading of “atypical pneumonia,” are atypical enough to justify their inclusion here.
2.2 General Features
Infections with many, if not all, atypical organisms share some common features. Mycoplasma, chlamydia, and viruses have many similarities in their pathology, clinical, and radiological features. Grossly, these pathogens cause predominant histological changes in the respiratory epithelium and in the peribronchial interstitium (Müller et al. 2007; Müller 2003). The ensuing bronchiolitis and alveolitis are characterized by infiltration of the epithelium with mononuclear cells and presence of neutrophilic exudate in the lumen of the airways. These changes are predominantly seen in the terminal and respiratory bronchioles and manifest radiologically as peribronchial nodules (Müller et al. 2007; Müller 2003).
Bronchiolitis with exudate in the lumen of the airways is seen on radiographs as reticulonodular pattern and on CT as tree-in-bud nodules. As the nodules enlarge, they involve the entire secondary pulmonary lobule and are seen as “lobular” consolidation: these features are typical of bronchopneumonia pattern. Further extension into segmental and patchy lobar consolidation may be seen in advanced stages, and this may be difficult to distinguish from bacterial infection. Partial filling of the airways gives rise to ground-glass opacities (Müller et al. 2007).
Some infections like influenza and hantavirus cardiopulmonary syndrome, on the other hand, cause rapidly progressive pneumonia with diffuse alveolar damage (Kim et al. 2002).
The clinical course of atypical pneumonias is generally subacute, the WBC counts are generally normal, and the imaging features are heterogeneous and nonspecific.
2.3 Radiological Aspects
Diagnosis of pneumonia requires a combination of clinical, microbiological, and radiological features. Chest radiograph is usually sufficient to confirm the diagnosis. It also helps in follow-up of the patients and evaluation of the response to treatment. CT scans are not routinely recommended but are helpful in case of doubtful features on chest radiographs, when no adequate response to the treatment is noted and when complications or underlying pathology is suspected (Vilar et al. 2004b). CT scan can be further helpful in suggesting an etiological diagnosis. In indeterminate cases, CT-guided procedures come in as problem-solving tools for the correct diagnosis.
2.4 Bacteria
2.4.1 Mycoplasma Pneumonia
Mycoplasma pneumoniae is a common cause of community-acquired pneumonia, particularly in young children. It is the smallest free-living organism, measuring approximately 125–150 microns in size which is similar to that of myxovirus (Marrie et al. 2012). Lack of cell wall makes them highly pleomorphic.
Mycoplasma pneumoniae was first isolated from the sputum of a patient with “atypical pneumonia” by Eaton et al. and was initially considered to be a virus due to its ability to pass through bacterial filters. Subsequent studies led to its identification as bacteria (Cunha 2010).
Mycoplasma pneumonia is commonly seen in young children and adolescents and shows a declining incidence in adults (Meyer Sauteur et al. 2016). It spreads through droplet spread or direct contact. The incubation period is around 1–2 weeks (John et al. 2001).
Clinical symptoms resemble a viral infection and are characterized by mild symptoms like dry cough, headache, malaise, and low-grade fever (John et al. 2001). In view of these mild symptoms, it is also called as “walking pneumonia” (Meyer Sauteur et al. 2016). However, sometimes severe infection and extrapulmonary manifestations may also be seen. In one study by Marrie et al., common extrapulmonary manifestations were thrombocytosis, hemolysis, Guillain-Barré syndrome, and pulmonary hemorrhage (Marrie et al. 2012). In asthmatic patients, it can cause an acute exacerbation of asthma and present with acute dyspnea.
Typically, the WBC counts remain in the normal range. A secondary bacterial infection is likely when they are elevated (John et al. 2001).
An attempt to suggest the offending organisms on radiology can be worthwhile for two main reasons: firstly, infection with these cell wall-deficient bacteria calls for treatment with antibiotics other than the usual cell wall synthesis inhibitors like penicillin, which are otherwise suitable for typical infections with pneumococcus. Laboratory tests take time, pending, which a radiological diagnosis can prove helpful in clinical decision making. Secondly, in appropriate settings, radiological features, although not highly specific, can be suggestive of the diagnosis in many cases.
The chief pathology is acute cellular bronchiolitis with bronchial wall inflammation, peribronchial infiltrates of lymphocytes and macrophage, and intraluminal exudative fluid (Pipavath et al. 2005; Muller and Miller 1995). This is seen radiologically as thickening of bronchial wall and centrilobular nodules.
Radiographic features of mycoplasma are generally nonspecific and resemble viral pneumonias closely. Reticulonodular infiltrates in one or more lobes are seen on radiographs (John et al. 2001) (Fig. 20). Various studies have highlighted lower lobe predominance (John et al. 2001; Guckel et al. 1989). Bilateral peribronchial infiltrates may also be seen. Patchy consolidation may be seen; however, dense lobar consolidation as in typical bacterial pneumonias is less likely (Eaton et al. 1944).
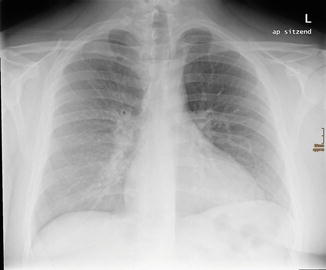

Fig. 20
A 21-year-old man with mycoplasma pneumonia: chest radiograph showing enlarged left hilar shadow. Reticulonodular opacities in the right lower lung field
Mycoplasma pneumoniae pneumonia presents itself on computed tomography (CT) like bronchopneumonia. Centrilobular nodules are the most common findings on CT (Reittner et al. 2000b) (Fig. 21). They coalesce and form consolidations. Consolidations and ground-glass opacities with patchy involvement of the secondary pulmonary lobules and interspersed sparing of some lobules are typical (Reittner et al. 2000b). A combination of bronchial wall thickening and centrilobular nodules with bronchopneumonia can be highly suggestive of mycoplasma. CT helps to demonstrate the nodules and bronchial wall thickening better than radiographs, thereby helpful in pointing toward the diagnosis. Pleural effusions are less commonly encountered, seen in around 20 % of the cases, and when present are transient (John et al. 2001; Hsieh et al. 2007).
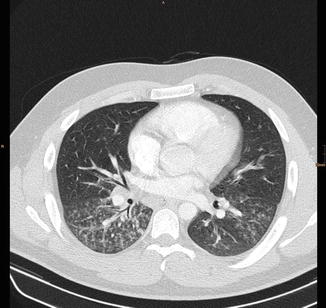

Fig. 21
Same patient as in Fig. 20: CT scan showing centrilobular nodules, tree-in-bud, and bronchial wall thickening in both lower lobes
2.4.2 Chlamydia pneumoniae
Chlamydias are obligate intracellular bacteria that possess a cell wall. They exist in two forms: a smaller extracellular elementary body and a larger intracellular form called the reticulate body (Webb et al. 2011). Three species of chlamydia area known:
Chlamydia trachomatis typically causes ocular infection (trachoma) and sexually transmitted disease but may cause pneumonia in infants who are born to infected mothers.
Chlamydia psittaci is a pathogen in birds (causing psittacosis) and may cause respiratory infections in humans. The mode of infection is through aerosols containing bacteria generated from infected birds (Knittler and Sachse 2015).
Chlamydia pneumoniae was the third species to be discovered, first isolated in 1968 during trachoma vaccine trail and accounts for an estimated 10 % of the CAP cases (Kuo et al. 1995). It is a common cause of atypical pneumonia.
Transmission of infection occurs through respiratory secretions. Incubation period is several weeks.
Symptoms tend to be protracted and are subacute in onset. Prolonged cough and low-grade fever are typical features. Initial prodromal phase of upper respiratory tract symptoms like pharyngitis and hoarseness of voice are not uncommon.
The infection is typically mild, and extrapulmonary symptoms are less common in comparison to mycoplasma infection. It has also been implicated in coronary artery disease.
CT features consist of bronchopneumonia with consolidation, GGO, peribronchial thickening, and nodules, similar to mycoplasma infection. However, pleural effusions are more common in patients with Chlamydia pneumoniae, bronchial wall thickening and centrilobular nodules more common in mycoplasma infection (Okada et al. 2005). Some studies described increased prevalence of emphysema and bronchial dilatation in Chlamydia pneumoniae pneumonia; COPD is an important risk factor for chlamydia (Hahn 1999; Karnak et al. 2001).
2.4.3 Legionella pneumophila
Legionella pneumonia is an atypical pneumonia, first discovered in 1976 due to an outbreak in a convention in Philadelphia (Fraser et al. 1977). The source of infection was later narrowed down to the cooling system of the air conditioner with circulating water which acts like a conducting medium for the bacteria.
It is a Gram-negative obligate intracellular bacterium that is a parasite of freshwater protozoans. It tends to colonize in various water facilities and causes outbreaks of infection when aerosols containing the bacteria are inhaled (Fields et al. 2002). In humans, it infects the macrophages, where it resides and multiplies.
Legionella pneumophila is described to cause two diseases: Legionnaires’ disease which is a severe multisystemic infection and pneumonia with a long incubation period from 2 to 10 days and Pontiac fever which is a mild clinical illness with flu-like symptoms and a short incubation period of 36 h. Legionella pneumophila serogroup 1 accounts for most of the cases (Fields et al. 2002).
Legionnaires’ disease varies from mild to severe disease requiring ICU care. It tends to cause electrolyte abnormalities, typically hyponatremia and hypophosphatemia, the presence of which must raise the suspicion of this infection. Presenting symptoms are usually constitutional like fever, myalgias, headache, confusion, and diarrhea which are seen early in the course of the disease, and then nonproductive cough appears later (Tsai et al. 1979).
Radiological features are usually nonspecific and resemble bacterial lobar pneumonia.
Radiographs show patchy peripheral consolidations initially. Airspace consolidation due to alveolitis is typical. Bilateral, multilobar, and multisegment consolidation and ground-glass opacities are the most common radiological features on CT (Yagyu et al. 2003). Centrilobular nodules and diffuse peribronchial thickening characteristic of mycoplasma and chlamydia are less commonly encountered in legionella (Kim et al. 2007). Cavitation may be seen occasionally in immunosuppressed individuals and patients on high steroids (Fairbank et al. 1991). Pleural effusions are seen in up to half of the patients and are usually mild or moderate. Delayed clearing up of the infiltrates and residual fibrosis may occasionally be seen.
2.5 Viruses
Lower respiratory tract infections can be caused by various viruses, and it is estimated that approximately 10 % of infections in CAP are due to viral etiology (ref). Along with the various radiological features, knowledge of the clinical history can be invaluable in narrowing the differential diagnosis. For example, in immunocompetent host, influenza virus is commonly implicated as an offender. In immunosuppressed individuals, however, the usual inciting organisms are herpes, cytomegalovirus, and adenovirus (Kim et al. 2002).
Viruses can be divided into (a) RNA viruses, myxoviruses (influenza, RSV, measles), coronavirus, and hantavirus, and (b) DNA viruses, adenovirus and herpes group including CMV and varicella zoster.
2.5.1 Influenza Virus
Influenza virus belongs to the family Orthomyxoviridae and is a single-stranded RNA virus. It has three predominant serotypes: influenza A, B, and C. Influenza A is responsible for majority of the epidemics and pandemics. Influenza virus A and B may cause pneumonia, while C causes only sporadic mild infections (Webb et al. 2011).
Infection with influenza virus is usually self-limiting. It favors secondary bacterial infection through various mechanisms like altering the respiratory epithelium. Diabetes mellitus, pregnancy, extremes of age, immunosuppression, and underlying pulmonary or cardiac conditions are some of the factors that predispose to a fulminant infection and pneumonia, and death within 24 h may be seen in extreme cases (Oikonomou et al. 2003). Such an infection is characterized histologically by diffuse alveolar damage.
Radiological features are varied and consist of segmental areas of consolidation which may be unilateral or bilateral (Fig. 22). Pleural effusions are rare. On CT, ground-glass opacities, centrilobular tree-in-bud nodules, and patchy consolidations may be seen (Oikonomou et al. 2003).
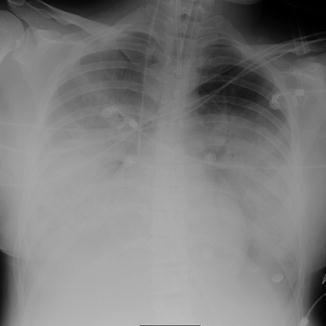

Fig. 22
A 29-year-old man with H1N1 pneumonia: chest radiograph showing extensive areas of consolidation in both lungs with only a relative sparing of the left upper lung field
2.5.2 Respiratory Syncytial Virus
It is an RNA virus belonging to Paramyxoviridae family. Almost all infants are infected with RSV by 2 years of age (Drysdale et al. 2016). RSV typically causes upper respiratory tract infections, otitis media, bronchiolitis, asthma-/viral-induced wheezing, and pneumonia. Bronchiolitis may be seen in up to 80 % of the cases (Drysdale et al. 2016). In immunocompromised individuals, it can cause severe pneumonia with high mortality (Mayer et al. 2014).
In addition to bronchial wall thickening and peribronchial nodules, typical features of RSV infection on radiography are air-trapping and hyperinflation due to bronchiolitis (Müller et al. 2007). CT reflects the features of bronchopneumonia and bronchiolitis. Multifocal consolidations/GGO with tree-in-bud nodules may be seen interspersed with areas of air-trapping (Mayer et al. 2014).
2.5.3 Measles
It is a febrile illness with rash caused by an RNA virus belonging to Paramyxoviridae family. It spreads from one person to another through droplet spread. The clinical manifestations are more severe in adults and immunocompromised hosts.
Bronchial wall thickening, centrilobular nodules, GGO, and interlobular septal thickening are seen on CT, and the features are nonspecific (Franquet 2011).
2.5.4 SARS-Coronavirus
Coronaviruses, commonly implicated in cold, are RNA viruses of Coronaviridae. In 2002, a new strain caused an outbreak in China which spread rapidly throughout the world (Peiris et al. 2003). This was called SARS-coronavirus (severe acute respiratory syndrome). Transmission occurs through droplet spread; incubation period varies from 2 to 14 days (Peiris et al. 2003).
Typical symptoms are fever with myalgia and cough. Characteristic early CT findings include focal ground-glass opacities and crazy-paving pattern which are scattered in distribution. Subsequent development of consolidations may be seen. Air leak syndromes with development of spontaneous mediastinum or pneumothorax have also been reported. Residual fibrosis and scarring may also be seen (Chan et al. 2004).
2.5.5 Hantavirus
Hantaviruses are a group of RNA viruses belonging to the family Bunyaviridae (Mattar et al. 2015). Unlike other infections of Bunyaviridae, infection with hantaviruses is a rodent-borne zoonosis that spreads through inhalation of rodent secretions (feces, urine, or saliva), and humans are accidental hosts (Manigold and Vial 2014). Each species of hantavirus is associated with a unique rodent host, and thus the epidemiology of hantavirus infections correlates closely with the geographical distribution of the respective rodents (Manigold and Vial 2014).
Infection with hantaviruses causes two distinct clinical pictures: hemorrhagic fever with renal syndrome (HFRS) which is more common in Europe and Asia, caused by the “old world” hantaviruses, and hantavirus cardiopulmonary syndrome (HCPS) which is more common in the Americas, caused by the “new world” hantaviruses (Mattar et al. 2015; Manigold and Vial 2014). Close to 150,000–200,000 cases occur every year, commonly in Asia (Manigold and Vial 2014). In Germany, a peak in 2012 with more than 2800 new cases was reported (Kruger et al. 2013).
Pathologically both these infections are characterized by breakdown of the endothelial barrier in blood vessels and marked vasodilatation.
HFRS is characterized by fever followed by hypotension and petechial hemorrhages due to vascular leakage, oliguric phase, polyuric phase, and convalescence. Oliguric phase is the most critical phase characterized by renal failure and pulmonary edema, and most of the case fatalities occur in this stage.
After an incubation period of 9–35 days, HCPS is characterized by abrupt onset of fever and other constitutional symptoms. In the next cardiopulmonary phase, cough, dyspnea, tachycardia, and hypotension occur due to extravasation of fluids as a result of increased capillary leakage. Rapidly progressive noncardiogenic pulmonary edema and cardiogenic shock may be seen with respiratory failure and need for ventilator support. Case fatality rate is high (Manigold and Vial 2014).
Chest radiography shows features of permeability edema: in mild cases, pleural effusions and interstitial infiltrates are seen (Fig. 23). In severe cases, marked alveolar edema with predominance in the perihilar and basal regions and sparing of the periphery is typical (Boroja et al. 2002). CT shows extensive GGO in middle and lower zones with thickening of the septa (Gasparetto et al. 2007).
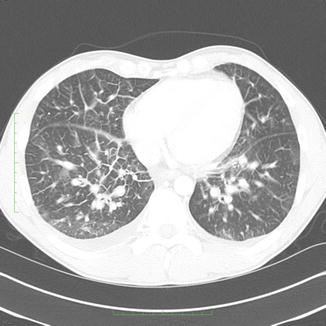

Fig. 23
A 32-year-old man with hantavirus infection: CT showing extensive thickening of the septa, areas of ground-glass, and minor pleural effusions
2.5.6 Adenovirus
They are DNA viruses belonging to Adenoviridae family. Many serotypes exist.
Adenovirus is a common cause of upper respiratory tract infection in children. Severe infection is seen in immunocompromised patients. It causes sequelae such as bronchiectasis, obliterative bronchiolitis, and Swyer-James syndrome (unilateral hyperlucent lung).
Pathologically, severe adenovirus pneumonia is characterized by areas of hemorrhagic consolidation, interspersed with air-trapping, atelectasis, and diffuse alveolar damage (Becroft 1967). In mild cases, inflammatory cell infiltrate in the alveoli and interstitium is seen (Chong et al. 2006).
Radiographs show unilateral or bilateral parenchymal opacities. Especially in children, areas of overinflation and atelectasis may be seen (Kim et al. 2002). On CT, extensive GGO with or without consolidations are noted. GGO correlates with areas of diffuse alveolar damage. In a study by Chong et al. in five adults with adenovirus pneumonia, one patient had shown crazy-paving appearance (Chong et al. 2006).
2.5.7 Herpes Simplex Type 1
Herpes simplex type 1 is a DNA virus of the Herpesviridae, along with CMV and varicella zoster virus. Primary infection is usually asymptomatic, but can manifest as pharyngitis or gingivostomatitis. Following initial infection, it remains latent and can undergo reactivation at a later stage and manifest as esophagitis or tracheobronchitis.
Pneumonia due to HSV-1 occurs commonly due to contiguous spread from upper respiratory tract or aspiration and less commonly due to hematogenous spread (Graham and Snell 1983; Ramsey et al. 1982). HSV-1 pneumonia is almost exclusively seen in immunosuppressed individuals or in patients with squamous metaplasia of the airways due to intubation, burns, or chronic smoking (Ramsey et al. 1982).
Fever, dyspnea, chest pain, and productive cough are usual clinical features. Herpes labialis and extensive oropharyngeal lesions may also be seen (Simoons-Smit et al. 2006).
The epithelial lesions are characterized by ulceration and necrosis. Pneumonia shows alveolar necrosis and exudate with inflammation (Kim et al. 2002).
2.5.8 Cytomegalovirus
Cytomegalovirus (CMV) is a member of herpes family and is a DNA virus. It is well known to cause opportunistic infections in immunosuppressed individuals especially in bone marrow or solid organ transplant recipients and AIDS patients.
Clinical features include fever, cough, and dyspnea.
The pathological features of CMV infection in transplant recipients are reported to be different from those seen in AIDS patients. In transplant recipients, predominant necrotizing pneumonia is seen due to immune-mediated mechanisms, and CMV pneumonia is seen in the first few months after transplantation. AIDS patients, on the other hand, have insufficient immune response and show effects primarily due to the cytopathic effect of the virus with diffuse alveolar damage (Kim et al. 2002).
Radiographic manifestations are nonspecific and consist of reticulonodular pattern and/or airspace consolidations. CT features consist of diffuse or focal ground-glass opacities (GGO), multiple nodules, and lobar consolidation. Nodular lesions with surrounding GGO (halo sign) may be occasionally seen, GGO representing hemorrhage or inflammation (Franquet et al. 2003). Dense consolidation or mass-like opacities may be seen in AIDS patients especially with Kaposi’s sarcoma (McGuinness et al. 1994).
2.5.9 Varicella Zoster
Varicella zoster is another virus belonging to Herpesviridae group. It causes two major manifestations: chickenpox (varicella) and herpes zoster. Pneumonia occurs most commonly in patients with chicken pox, although it may be seen in both forms (Müller et al. 2007).
Chickenpox is usually seen in children 2–8 years old; however, in recent times, the incidence in adults is increasing due to various factors (Mohsen and McKendrick 2003). While the common varicella infection in children is usually benign, infection in adults has a more fulminant course with a 25 times increased risk of pneumonia and consequent high mortality (Mohsen and McKendrick 2003).
Predisposing factors for the development of varicella pneumonia are immunosuppression, chronic lung diseases, pregnancy, or underlying malignancy like leukemia or lymphoma (Müller et al. 2007). Varicella pneumonia presents about 1–6 days after the onset of rash with cough, dyspnea, fever, and sometimes chest pain and hemoptysis (Mohsen and McKendrick 2003).
The pulmonary lesions in varicella seem to be due to hematogenous spread rather than airborne entry. The lesions are characterized by endothelial damage in pulmonary vessels with focal hemorrhagic necrosis, mononuclear infiltrates in the alveolar walls, and exudates in the alveoli (Mohsen and McKendrick 2003). Later small nodules are seen which are characterized by outer lamellated fibrous capsule and inner necrotic or hyalinized collagen with varying degrees of calcification (Kim et al. 1999).
On radiographs multiple, fleeting 5–10 mm miliary nodules are seen with a tendency to coalesce (Fig. 24). These resolve usually in a week after the regression of the skin lesions, but may also be persistent for months in some patients (Sargent et al. 1966). On CT bilateral nodules of soft tissue density measuring 5–10 mm are seen (Fig. 25). Some nodules show GGO around them, while some may coalesce with adjacent nodules (Kim et al. 1999). Hilar lymph nodes and pleural effusions are less commonly seen. Dense, calcified random dense nodules measuring 2–3 mm in size may persist in some cases.
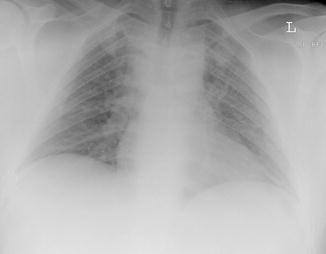
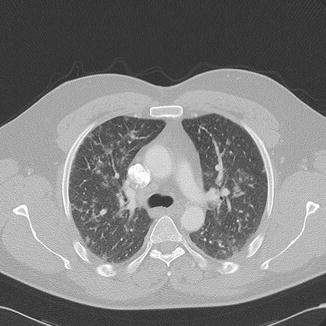

Fig. 24
A 35-year-old man with varicella virus infection: chest radiograph showing multiple fleeting 5–10 mm miliary nodules in both lungs

Fig. 25
Same patient as in Fig. 24: CT showing bilateral 5–10 mm nodules of soft tissue density and a ground-glass halo
2.6 Conclusion
Although radiography and CT can be helpful in excluding a CAP, they may not be accurate in suggesting an etiological diagnosis. In every case, careful attention to the clinical history and radiological findings may help to narrow down the etiology and suggest the diagnosis.
3 Tuberculosis
3.1 Introduction
Tuberculosis is often a challenge to the clinicians and radiologists alike, particularly in areas where it is not endemic. As the clinical signs and symptoms are often nonspecific, extra attention and a high degree of suspicion are required to make the right diagnosis.
As the manifestations of tuberculosis are a result of the interaction between the offending bacteria and the host immune system, there is no definite radiological picture that defines or is typical for tuberculosis and a wide range of differential diagnoses exist. However, some features, particularly when seen in typical distributions and typical combinations, can be very helpful in suggesting the right diagnosis, as discussed below.
3.2 Background
Tuberculosis (TB) or captain of all these men of death is one of the oldest diseases known to mankind. It is still a disease of global concern, affecting millions of people worldwide every year. It is a leading cause of death among infectious diseases. Together with HIV, TB accounted for a staggering 1.5 million deaths in 2014 (World Health Organization 2015). Ninety-five percent of these deaths were reported in low- to middle-income countries, where tuberculosis is highly prevalent (World Health Organization 2015).
Although the incidence of TB globally has been showing a declining trend for several years, still an estimated 9.6 million new cases were reported by WHO in 2014. Majority of these cases were reported in Southeast Asia, Western Pacific, and Africa with highest number of incident cases seen in India, Indonesia, and Nigeria. 12 % of these patients were HIV positive, and notably 75 % of HIV-positive people with tuberculosis lived in Africa (World Health Organization 2015).
Europe accounts for 4 % of TB burden in the world with close to 1000 new cases per day (European Centre for Disease Prevention and Control 2015). Within the EU/EEA (European Union/European economic area), an overall declining trend in TB reporting in the last 10 years has been observed. It is noteworthy that a significant 28 % of cases were of foreign origin. In some countries like Luxemburg, Malta, Norway, Sweden, and Switzerland, the TB cases of foreign origin accounted for >70 % cases, highlighting the increased prevalence in migrants, with an increased prevalence of MDR-TB in these groups of patients (European Centre for Disease Prevention and Control 2015; Odone et al. 2015).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree