Intracranial Infection
L. Celso Hygino da Cruz Jr
Introduction
Despite advances in antibiotic therapy, infectious diseases continue to be a threat to life and livelihood. Central nervous system (CNS) infections lead to persistent health problems that affect a significant percentage of the population worldwide. In brain infection in particular, prognosis depends on the prompt etiologic diagnosis and the correct treatment at presentation. Both early detection and accurate diagnosis of CNS infection are especially critical because most of these disorders are readily treatable yet highly destructive if treatment is delayed. When the appropriate clinical information is communicated to radiologists they can be of great importance in helping clinicians to narrow the spectrum of possible diagnoses. Beyond ascribing an infectious etiology to a patient’s abnormal scan, there are sometimes imaging clues that should suggest specific infectious agents. In this chapter, these findings are highlighted in the context of describing magnetic resonance imaging (MRI) appearances of intracranial infection. Whenever appropriate, we discuss the contribution of advanced neuroimaging techniques to the evaluation and diagnosis of specific pathologic entities.
Viral Infection
Intracranial viral infection is usually a multifocal or diffuse inflammatory process, termed encephalitis. In many cases, viral infection involves the meninges as well; it is then known as a meningoencephalitis. The disease process may result from acute or latent infection of the CNS.
In the general population, aseptic meningitis is most often due to enteroviruses (especially coxsackieviruses and echoviruses) and less frequently due to herpes simplex virus (HSV) or mumps virus. Viruses that may result in meningitis in AIDS patients include human immunodeficiency virus (HIV) and the herpes viruses, especially cytomegalovirus (CMV) and occasionally HSV types 1 and 2 (HSV-1 and HSV-2). Imaging is not highly sensitive to viral meningitides when the parenchyma is spared, even with postcontrast fluid-attenuated inversion recovery (FLAIR) imaging (the most sensitive MR sequence for meningitis, surpassing contrast-enhanced T1 images). Therefore, a normal MRI cannot totally exclude viral meningitis, and if confirmation of that diagnosis is sought beyond clinical examination and history, cerebrospinal fluid (CSF) analysis is indicated.
Pathologically, the primary features of viral encephalitis include neuronal degeneration and inflammation. Gross histopathologic findings range from unremarkable to diffuse brain congestion and edema with hemorrhage and necrosis (as in HSV-1, HSV-2, and some arboviral encephalitides). There is often some degree of cerebral edema and congestion of meningeal vessels.
On MR, the pathologic changes that result from viral encephalitis appear as scattered or confluent areas of hyperintensity on T2-weighted images and are isointense or hypointense on T1-weighted images, with variable mass effect. Foci of subacute hemorrhage (extracellular methemoglobin) demonstrate increased signal intensity on both T1- and T2-weighted images. Contrast enhancement may or may not be present. Diffusion-weighted imaging (DWI) characteristically shows patchy restricted diffusion and is key to the consideration of any acute encephalitis. On follow-up, localized or generalized atrophy resulting from chronic or prior infection with tissue destruction may appear. Although these general features apply to most of the viral encephalitides, certain infections demonstrate particular features that may be characteristic and are thus helpful in the differential diagnosis. These are described in the following subsections.
CNS Infections of Herpesvirus Family
The herpesvirus family consists of a large group of DNA viruses, where humans are the sole reservoirs for them. This family include HSV-1 and HSV-2, varicella zoster virus (VZV), CMV, Epstein–Barr virus (EBV) and herpes virus type 6 (HHV-6) and type 7 (HHV-7).
Herpes Simplex Virus Type 1
HSV-1 is the causative agent in more than 90% of herpetic encephalitis cases and it accounts for 10% to 20% of all viral encephalitis. Incidence is one to four cases per million people annually. HSV encephalitis (HSE) is the most common cause of fatal sporadic encephalitis and has a high mortality, ranging from 50% to 70% without treatment. Early diagnosis and initiation of appropriate treatment is of great importance. More than 70% of HSV-1 encephalitis cases result from reactivation of latent HSV-1 infection of the trigeminal ganglion in adult individuals previously exposed to the virus. The virus then spreads along branches of cranial nerve V that innervate the meninges of the anterior and middle cranial fossa (1). About one-third of herpes encephalitis cases are due to the primary HSV infection.
A nonspecific alteration in mental status, including a lowered level of consciousness, together with focal neurologic deficit, seizures, and fever are the principal clinical symptoms in adults. The diagnosis of HSE should be considered in a patient with a progressively deteriorating level of consciousness, fever, focal neurologic findings, in the absence of other causes. CSF findings are nonspecific, and isolation of HSV from the CSF is rare. However, polymerase chain reaction (PCR) techniques are routinely employed in obtaining a more accessible and rapid diagnosis from the CSF, reducing the necessity of brain biopsy. A single negative PCR result does not exclude the diagnosis,
especially if it was performed within the first 72 hours after onset of clinical symptoms (2). Early intervention with appropriate treatment is important because it can dramatically improve patient outcome. Therefore, the radiologist must be cognizant of the characteristic neuroanatomic pattern of involvement in HSE and specifically suggest the diagnosis when that pattern is identified. In such cases, treatment is instituted immediately (3).
especially if it was performed within the first 72 hours after onset of clinical symptoms (2). Early intervention with appropriate treatment is important because it can dramatically improve patient outcome. Therefore, the radiologist must be cognizant of the characteristic neuroanatomic pattern of involvement in HSE and specifically suggest the diagnosis when that pattern is identified. In such cases, treatment is instituted immediately (3).
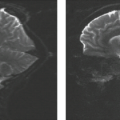
Herpes encephalitis is often but not always hemorrhagic, affecting primarily the medial temporal and inferior frontal lobes. In most cases, herpes encephalitis is initially unilateral, with asymmetric contralateral involvement seen in later stages. The most suggestive finding to be sought by the radiologist is unilateral or bilateral involvement of the insula in association with other frontal and temporal cortex (Fig. 14.1). Despite
the fact that this is a limbic inflammation, commonly affecting cingulate and parahippocampal gyri, the diagnostic radiologist should note that it would be highly atypical for herpes encephalitis to involve the hippocampus alone, that is, without any insular and temporal cortex changes. This stands in contrast to other disease states affecting hippocampi (including limbic encephalitis, a paraneoplastic noninfectious process, and status epilepticus), which can be isolated to unilateral or bilateral hippocampus. In immunocompetent individuals, HSV-1 encephalitis may result in necrotizing encephalitis involving mostly the temporal lobes and orbital surfaces of the frontal lobes. Other locations are also involved. The limbic system, including the insular cortex, cerebral convexity, cingulate gyrus, and posterior occipital cortex, may become involved. The disease often spares the basal ganglia. As noted, lesions strictly limited to the hippocampus should prompt the consideration of other entities. Although disease is not found with higher prevalence in immunocompromised patients, the presentation and pattern of involvement are more variable in that setting.
the fact that this is a limbic inflammation, commonly affecting cingulate and parahippocampal gyri, the diagnostic radiologist should note that it would be highly atypical for herpes encephalitis to involve the hippocampus alone, that is, without any insular and temporal cortex changes. This stands in contrast to other disease states affecting hippocampi (including limbic encephalitis, a paraneoplastic noninfectious process, and status epilepticus), which can be isolated to unilateral or bilateral hippocampus. In immunocompetent individuals, HSV-1 encephalitis may result in necrotizing encephalitis involving mostly the temporal lobes and orbital surfaces of the frontal lobes. Other locations are also involved. The limbic system, including the insular cortex, cerebral convexity, cingulate gyrus, and posterior occipital cortex, may become involved. The disease often spares the basal ganglia. As noted, lesions strictly limited to the hippocampus should prompt the consideration of other entities. Although disease is not found with higher prevalence in immunocompromised patients, the presentation and pattern of involvement are more variable in that setting.
Although computed tomography (CT) may be negative in the early phase of disease, it can reveal hypodense lesions in the temporal lobes with or without involvement of the frontal lobes. Enhancement and gross hemorrhage are infrequent on CT. MRI techniques are far more sensitive than CT in demonstrating parenchymal involvement; however, some authors have noted negative MRI in almost 10% of patients with CSF PCR-positive (1). MR demonstrates the early edematous changes of herpes encephalitis, with increased signal and swelling seen in the temporal and inferior frontal lobes on T2-weighted images. This hyperintense signal involves both cortex and white matter and may be seen within hours after the onset of signs and symptoms, compared with the reported delay of up to 3 to 5 days on CT.
MR often demonstrates bitemporal involvement when CT shows only unilateral infection. As areas of involvement enlarge and coalesce, the early associated mass effect may increase. Extension from the temporal lobes across the Sylvian fissure to the isle of Reil is frequently seen, sparing the putamen. Enhancement is often absent or only minimal in the early stages (Figs. 14.1 and 14.2), but cortical enhancement can be seen immediately and often becomes more prominent with disease progression (Fig. 14.3). Magnetization transfer (MT)-suppressed T1-weighted images may be able to depict subtle areas of enhancement, but visualization of enhancement is not essential for making the correct diagnosis. Perfusion MRI, such as cerebral blood flow (CBF) or cerebral blood volume (CBV) mapping, can show high blood flow or blood volume even though no contrast enhancement is seen (Fig. 14.1). However, characteristically infection processes demonstrate decreased perfusion. Focal hemorrhage is consistently present at autopsy yet is often not detected on early imaging studies obtained in the acute phase of the disease. Atrophy and clear parenchymal destruction with old hemorrhage is a common late sequela well visualized on follow-up MR (Fig. 14.4). MR has been used to monitor response to treatment with acyclovir.
It has been suggested that the pattern of HSV-1 involvement in infants and young children differs from that of encephalitis in adults, older children, and neonates. In infants and young children particularly, extensive bilateral cortical areas of the hemispheres and the adjacent subcortical white matter may be involved at the beginning of the disease (Fig. 14.5). Distribution of involved areas sometimes corresponds to a vascular distribution, suggesting a hematogenous spread. Diffuse cortical swelling involving the occipitoparietal cortex and subcortical white matter, thalamus, and corpus callosum can also be demonstrated (3).
As in all encephalitides, DWI shows characteristic findings of patchy restricted diffusion acutely. This is helpful in distinguishing encephalitis from other lesions. In HSE, this may be particularly important because the neuroanatomic pattern may be mimicked by noninfectious entities that also present with seizures in the same patient population, such as neoplasm. Herpes encephalitis typically shows heterogeneously restricted diffusion in early stages of disease, when the inflammatory reaction is greatest (Figs. 14.1, 14.2, and 14.6) (4). In some circumstances, DWI is able to depict parenchymal alterations characterized by restricted diffusion before abnormalities are noted on T2-weighted and FLAIR images. DWI may reveal a greater extent and number of lesions than T2-weighted images and may be a method for monitoring treatment response (5). Later, parallel to the development of vasogenic edema and encephalomalacia, elevated diffusion with high apparent diffusion coefficient values is seen. In the acute phase, MR spectroscopy (MRS) demonstrates a reduction of N-acetylaspartate (NAA) levels at the temporal lobes, reflecting a neuronal loss secondary to herpes encephalitis, associated to a mild increase in choline level and lactate. Glutamine–glutamate complex may also be increased in acute stage. Elevation in myo-inositol (mI) may represent gliosis (Fig. 14.7).
Others areas of the CNS can be also involved concomitant without encephalitis. Cranial nerve involvement is not uncommon in herpes simplex infection (4).
HSE associated with HIV infections is a rare complication, occurring in 2% of AIDS autopsy cases, in patients with neurologic symptoms. In AIDS patients, HSV often results in diffuse rather than localized temporal/frontal involvement (Fig. 14.8). Mild to severe forms of both HSV-1 and HSV-2 encephalitis have been reported and may coexist with other infections. Typical pathologic findings of necrotizing encephalitis may
be absent in the AIDS patient, even when HSV-1 or HSV-2 is cultured from the brain tissue. In these immunocompromised patients, there appears to be an inverse relationship between the degree of immunodeficiency and the severity of the inflammation induced by the herpes viruses and the rapidity of disease progression.
be absent in the AIDS patient, even when HSV-1 or HSV-2 is cultured from the brain tissue. In these immunocompromised patients, there appears to be an inverse relationship between the degree of immunodeficiency and the severity of the inflammation induced by the herpes viruses and the rapidity of disease progression.
Key Points
Most common symptoms
Altered mental status
Focal neurologic deficit
Seizures
Fever
CSF, HSV, PCR positive
Imaging features
FLAIR and DWI most sensitive sequences;
T2WI and FLAIR: hyperintense insula/temporal cortex/subcortical white matter;
T1WI: may show hemorrhage;
DWI: patchy restricted diffusion (even with normal MRI);
CBV elevated, even without parenchymal enhancement.
Limbic system: temporal lobes, insula, inferior frontal lobes, Sylvian fissures, cingulated gyrus, and posterior occipital cortex.
Herpes Simplex Virus Type 2
HSV-2 is a major cause of neonatal encephalitis along with other TORCH (toxoplasmosis, other, rubella, CMV, and herpes) agents. The primary route of infection is via the maternal birth canal, although infrequent hematogenous transplacental in utero infection can occur. In children, HSV-1 acquisition is usually postnatal, and in neonates, HSV-2 acquired via maternal genital herpes is much more frequent than HSV-1, accounting for 80% to 90% of neonatal herpes virus infections and almost all congenital herpes virus infections. The CNS is involved in approximately 30% of infected infants (6). HSV-2 encephalitis presents with fever, seizures, abnormal CSF HSV PCR, abnormal MRI brain imaging, ventriculomegaly, multicystic encephalomalacia, and death (7). Patients that survive have neurodevelopmental problems, with psychomotor retardation, learning disabilities, microcephaly, microphthalmia, and blindness (7).
One in 2,000 to 5,000 neonates is infected per year. Approximately 35% of these develop encephalitis and 25% have the disseminated disease. Use of acyclovir may improve the outcome and has reduced mortality of HSV-2 encephalitis from 40% to 15% (7).
Pathologic examination demonstrates acute and chronic parenchymal and leptomeningeal inflammation, with a diffuse pattern of involvement that may result in widespread brain destruction.
Neuroimaging reflects various degrees of parenchymal inflammation and leptomeningeal involvement, evolving to necrosis. HSV-2 encephalitis usually localizes in the frontal and temporal lobes like HSV-1. Immunocompromised patients are not usually infected by HSV-2. When infected, these patients develop a subacute encephalitis, with a more diffuse involvement. In immunocompetent adults, HSV-2 can cause aseptic meningitis. MRI may reveal a diffuse meningeal contrast enhancement (4).
CT may reveal only subtle hypodensity of the periventricular white matter with relative sparing of the central gray matter, including basal ganglia and thalami, and the posterior fossa. This may progress to increased white matter lucency and a fingerlike increase in the density of the cortex. This augmented density is believed to result from an increase in cortical blood flow as a consequence of cortical infection. With disease progression, CT may show focal hemorrhagic necrosis, parenchymal calcification, and cystic encephalomalacia.
MRI findings are similar to those of CT in early stages of disease, including a loss of distinction at the gray–white matter interface. DWI can demonstrate restricted diffusion in the involved area at the acute phase. Edema may be difficult to distinguish from the surrounding unmyelinated immature white matter because both have increased signals on T2-weighted images. Reduced signal intensity on T2-weighted images of the cortices may be related to hemorrhagic necrosis and parenchymal calcifications.
Key Points
Major cause of neonatal encephalitis
Most common symptoms
Fever
Seizures
May lead to death
Psychomotor retardation
Microcephaly
Microphthalmia, blindness
Imaging features
T2WI and FLAIR: hyperintense cortex/subcorticala white matter
Leptomeningeal involvement
Focal hemorrhagic necrosis
Cystic encephalomalacia
Spare central gray matter
Varicella Zoster Virus
VZV can cause two distinct clinical disorders, an acute febrile exanthematous illness (varicella or chicken pox) and herpes zoster (shingles) infection. Although CNS infection may be seen in both entities, it is rare in healthy populations.
Varicella is a highly contagious generalized skin eruption that occurs primarily in children and usually produces no serious consequence in those who are healthy. In immunocompetent patients, encephalitis is seen in less than 1% of infected patients. Mortality is low, with complete or near-complete recovery as the usual outcome. In immunocompromised patients, however, encephalitis may result. Neurologic complications of VZV reactivation occur most frequently in the elderly and in immunocompromised individuals. Among AIDS patients, VZV infection is described in around 4% of infected people (8). The different patterns suggest that spread of VZV to the CNS can occur in different ways. Latent viruses residing in the ganglia of cranial nerves (especially cranial nerves V and VII) can reactivate and extend retrogradely to the brainstem by direct transneuronal spread, resulting in encephalitis. A hematogenous spread and CSF seeding, as opposed to the classically described “reactivation” of a dormant virus in the dorsal root ganglia that produces cutaneous shingles, have also been proposed.
Varicella CNS infection may result in transverse myelitis, meningoencephalitis, cerebellar ataxia, and aseptic meningitis. Symptoms of cerebellar ataxia are usually self-limited and often concurrent with the skin eruptions. Neuroimaging findings, however, are often negative in these patients. Meningoencephalitis is a serious uncommon CNS complication of varicella presenting with fever, headache, vomiting, seizures, and altered mental status some days to several weeks after the onset of the rash. CSF reveals mild to moderate lymphocytic pleocytosis and elevated protein. EEG may also be diffusely abnormal. On MR, multifocal areas of increased signal in the cortex have been observed on T2-weighted images.
Zoster CNS infection may produce encephalitis, neuritis, myelitis, and/or herpes ophthalmicus. These rarely complicate the clinical course in healthy adults with shingles, but in immunodeficient patients, there is an increased risk of CNS involvement. In immunocompetent patients, cranial and peripheral nerve palsies are the most common neurologic disorders seen in zoster infections, whereas diffuse encephalitis is the most frequent manifestation seen in AIDS patients and other immunosuppressed patients. Five different patterns of CNS involvement in AIDS patients have been proposed: multifocal leukoencephalitis, ventriculitis, acute meningomyeloradiculitis, focal necrotizing myelitis, and necrotizing angiitis involving leptomeningeal arteries with cerebral infarction. Fever, meningismus, and altered mental status in a patient with shingles suggest the diagnosis. CSF is nonspecific, with mild lymphocytic pleocytosis, slightly elevated protein, and normal glucose. The characteristic MR features of this infection are clustered subcortical plaquelike lesions demonstrating rapid demyelination and active lesions enhanced with intravenous contrast medium administration. Edema and hemorrhage are not prominent early findings but develop as the infection evolves. MR may also reveal an increased signal in the brainstem and supratentorial gray matter on T2-weighted images and may reveal brainstem enlargement despite meningeal enhancement (Fig. 14.9). The multifocal leukoencephalopathy pattern of VZV necrotizing encephalitis has a unique MR appearance and distribution, with multiple discrete target lesions coalescing into larger regions of extensive parenchymal involvement.
Cranial and peripheral nerve palsies occur in dermatomes affected by the distinguishing skin lesions. Involvement of cranial nerve V results in pain in the distribution of the trigeminal nerve, associated with headache and sometimes a change in the corneal reflex. The first division of cranial nerve V is the branch most often affected (herpes zoster ophthalmicus) and presents with pain and a vesicular eruption in the distribution of the ophthalmic division of cranial nerve V. Fat-suppressed MRI with gadolinium may show enhancement of the intraorbital portion of the trigeminal nerve. Contralateral hemiplegia may develop and is usually preceded by herpes ophthalmicus several weeks to months before. The immunodeficient patient is at increased risk for this complication. Pathogenesis of the hemiplegia is believed to result from viral infection of the larger intracranial arteries, resulting in cerebral angiitis and formation of mycotic aneurysms.
The Ramsay Hunt syndrome is characterized by the geniculate ganglion involvement, accompanied by seventh and eighth cranial nerve impairment, resulting in facial palsy, hearing loss with vertigo, and ipsilateral herpetic lesions of the external ear and canal. On MRI, this syndrome is represented by a contrast enhancement of the seventh and eighth cranial nerves.
Bickerstaff encephalitis is a rare monophasic inflammatory condition that affects the brainstem, probably caused by an immune-mediated response to VZV or CMV infection.
Clinical symptoms related to this conditions are ataxia, ocular paresis, and impaired reflexes.
Clinical symptoms related to this conditions are ataxia, ocular paresis, and impaired reflexes.
Reports associated VZV infection with arterial ischemic stroke and transient ischemic attack. A postvaricella arteriopathy is described, characterized by unilateral stenosing arteriopathy affecting the distal internal carotid artery and proximal segment of the anterior cerebral artery and middle cerebral artery. Thus, brain imaging almost always discloses infarcts within the vascular territory of their lenticulostriate branches, mostly involving basal ganglia and the vascular territory of the main cerebral arteries. CT may show hypodense lesions on basal ganglia, which are nonenhancing hypointense on T1-weighted images and hyperintense on T2-weighted images, corresponding to infarcts (9).
Diffuse encephalitis associated with VZV infection is rare. It is usually seen in immunocompromised patients, and the white matter may be more involved than the gray matter. Affected areas appear hyperintense on T2-weighted images and may be a result of direct infection and/or an immune-mediated reaction. Vasculitis may also occur.
Epstein–Barr Virus
EBV causes infectious mononucleosis that clinically presents with fever, lymphadenopathy, and eruption. EBV is associated with a number of CNS disorders, including acute encephalitis, meningitis, Guillain–Barré syndrome, meningoencephalitis or cerebellitis, cranial neuritis (mostly involving the second and seventh cranial nerves), transverse myelitis, and chronic fatigue syndrome. These may occur in the presence or absence of infectious mononucleosis. CNS complications are observed in approximately 5% of patients with infectious mononucleosis, including diffuse encephalitis—which has been seen in less than 1% of patients with infectious mononucleosis—demyelinating disease, acute cerebellar ataxia, myelitis, and meningitis (10). Diffuse encephalitis has a short but severe clinical course and a good prognosis for recovery. MRI findings related to EBV infection are not as common. Most acute cases do not reveal any abnormality. MR may reveal multifocal, diffuse, and reversible areas of hyperintensity on T2-weighted images in gray matter or at the gray–white junction. Basal ganglia, corpus callosum, and the brainstem may also be involved. Restricted diffusion has been noted in a lesion located in the splenium of the corpus callosum. This lesion showed reversible reduction in apparent diffusion coefficient after the patient’s improvement of symptoms (11). An unusual fusiform arterial dilation may be secondary to direct virus infection of vessels.
Cytomegalovirus
In adults, CMV infection is typically seen in AIDS patients, accounting for 85% of patients, and is less common, corresponding to 12%, in other immunocompromised patients and only 3% in immunocompetent individuals. This virus exists in a latent form in most of the population, and nearly 90% of adults have antibodies to CMV. Reactivation usually results in a subclinical or mild infection, mimicking mononucleosis. In some immunodeficient patients, however, reactivation results in disseminated infection and/or severe necrotizing meningoencephalitis and ependymitis. CMV may involve the CNS and/or
peripheral nervous system. CNS involvement is more common in the brain, but spinal cord lesions may also be noted. Neurologic manifestations of CMV include acute or chronic meningoencephalitis, cranial neuropathy, vasculitis, retinitis, myelitis, brachial plexus neuropathy, and peripheral neuropathy (4).
peripheral nervous system. CNS involvement is more common in the brain, but spinal cord lesions may also be noted. Neurologic manifestations of CMV include acute or chronic meningoencephalitis, cranial neuropathy, vasculitis, retinitis, myelitis, brachial plexus neuropathy, and peripheral neuropathy (4).
In AIDS patients, CMV frequently disseminates to CNS in the late stages with low CD4+ cell count. Usually, it may coexist with other lesions, including toxoplasmosis and cryptococcosis. This infection may be subclinical in immunocompetent and in immunocompromised patients. Approximately 70% of adult AIDS patients show CMV on neuropathologic examination (12).
Neuropathologic changes in CMV include atrophy, periventricular necrosis, neuronal loss, demyelination, and accumulations of enlarged cells with distended nuclei containing eosinophilic viral inclusions and surrounded by a halo, producing the characteristic “owl’s-eye” appearance (Fig. 14.10). Other typical histopathologic findings in the CNS include well-circumscribed microglial nodules. CMV intranuclear inclusions may also be found in the spinal cord, spinal nerves, and retina.
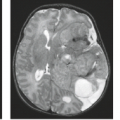
CMV can spread to the meninges and adjacent cranial nerve roots within the brain causing meningoencephalitis and ventriculitis (Fig. 14.11). It is most often seen in HIV-seropositive patients and transplant patients, but it can also occur in otherwise healthy adults. Less often, subacute symptoms develop over days to months, with fever, confusion, altered mental status, memory loss, and progressive dementia. Brain involvement may be diffuse or limited to the subependymal regions. CSF findings and complement fixation blood titers are nonspecific, and thus the clinical diagnosis can be difficult.
CT is virtually always less sensitive than MR in detecting CMV encephalitis abnormalities. CT grossly underestimates the degree of involvement by CMV and it is usually normal at the acute stage (12). Typically, CT may reveal atrophy, the most common finding, and, less commonly, white matter hypodensity and ring-enhancing lesions. In addition to atrophy and white matter hypodensity, periventricular and subependymal enhancement may be noted.
MRI depicts patchy and, less often, confluent periventricular white matter lesions with hypointense signal on T1-weighted images, as well as a hyperintense signal on T2-weighted images (Fig. 14.12). In less than 50% of the cases a thin (<2 mm; more often 1 mm), linear, characteristic subependymal contrast enhancement is evident and, if present, is a valuable diagnostic clue (4). Less frequently, hemorrhagic and necrotic lesions, micronodular encephalitis, and meningitis can be observed in the CMV infection (12). Fat-suppressed MR with gadolinium may reveal a thickened enhancing choroid/retina in patients with CMV retinitis, a hemorrhagic retinitis affecting 20% to 40% of AIDS patients. MR can also be used to follow treatment response.
MRS reveals an elevation in choline level and prominent lactate peak in acute stage of necrotizing encephalitis (13). The
lesion also has low relative cerebral blood volume (rCBV) on perfusion MRI when compared to normal brain parenchyma (4).
lesion also has low relative cerebral blood volume (rCBV) on perfusion MRI when compared to normal brain parenchyma (4).
CMV is the leading cause of congenital infection in developed countries and is the most common cause of serious fetal and neonatal encephalitis. CMV infection acquired in utero is a result of transplacental transmission from a primary maternal infection. From 30% to 40% of maternal primary infection cases result in fetal infection. Infected infants are often born prematurely (14). Only 10% of infants with congenital CMV are symptomatic at birth, with evidence of jaundice, thrombocytopenia, chorioretinitis, and hepatosplenomegaly (14). From 50% to 75% have microcephaly. In utero acquisition of CMV may result in intracranial parenchymal necrosis and hydrocephalus. Seizures, optic atrophy, sensorineural hearing loss, and mental retardation may become evident. From 10% to 15% of asymptomatic CMV-infected children and all individuals symptomatic at birth develop persistent neurologic disorders, such as neurologic impairment and hearing and vision deficits (15).
CT demonstrates atrophy, encephalomalacia, ventricular enlargement, porencephaly, subdural collections, multicystic encephalomalacia, and periventricular calcification, which can be demonstrated in 40% of cases and are more easily detected by CT. Periventricular calcification (mineralizing microangiopathy) and cystic encephalomalacia are also seen in other TORCH infections and are easily detected by neuroimaging. Calcifications of the cortex and basal ganglia are present less commonly but can also be seen in congenital CMV infections.
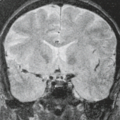
MR findings include dilated ventricules, enlarged subarachnoid spaces, gyral abnormalities, delayed myelination, and white matter lesions. MR also has the advantage of demonstrating migrational abnormalities that can result from the teratogenic effect of CMV infection. Migrational abnormalities are often associated with other evidence of congenital CMV infection and include the lissencephaly–pachygyria spectrum (Fig. 14.13).
In those patients with proven but neonatally asymptomatic congenital CMV infection, MRI may reveal cysts in the anterior portion of the temporal lobe and dilated inferior horns. A recent report also described white matter abnormalities consisting of multifocal lesions, mostly involving the deep white matter of parietal lobes and relatively sparing the immediately periventricular and subcortical white matter. In patients with polymicrogyria, both diffuse and multifocal white matter abnormalities have been described.
Human Herpesvirus Types 6 and 7
HHV-6 and occasionally HHV-7 cause exanthema subitum (roseola infantum) that affects children between 6 months and 2 years of age. In particular cases, children infected with HHV-6 can present febrile seizures. HHV-6 CNS infection is associated with subacute encephalitis, meningitis, meningoencephalitis, myelitis, and chronic fatigue syndrome. MRI demonstrates a selectively involvement of the temporal lobes, as high signal on T2WI, restricted diffusion, and a faint to prominent contrast enhancement. Involvement of the thalami, basal ganglia, cerebellar hemispheres, and brainstem are associated to acute necrotizing encephalopathy (ANE). Decreased perfusion within the lesion as well as overall decrease in all metabolites, except for lactate, is also noted (16).
HHV-7 is associated with meningoencephalitis and febrile seizures and hemiplegia. Necrotizing encephalitis may involve the brain stem, basal ganglia, and cerebellar and cerebral cortical areas.
Human Immunodeficiency Virus
HIV is a human retrovirus that causes AIDS. There are two subtypes, HIV-1 and HIV-2. Although HIV-1 is present all over the world, HIV-2 is found most frequently in West Africa. HIV has infected approximately 75 million people, causing an estimated 36 million deaths since the disease outbreak in 1981. It is estimated that 35.3 million people are infected with HIV, and almost 1.6 million people died from AIDS in 2012. Although only approximately 10% of the world’s population lives in sub-Saharan Africa, this area corresponds to almost 70% of the world’s HIV population, 25 million people, and 1.2 million AIDS-related deaths. At the end of 2012, the HIV epidemic in the United States had infected around 1.2 million people. The
introduction of highly active antiretroviral therapy (HAART) decreased the overall incidence of AIDS and substantially increased survival after AIDS diagnosis. However, disparities remain among racial/ethnic minority populations.
introduction of highly active antiretroviral therapy (HAART) decreased the overall incidence of AIDS and substantially increased survival after AIDS diagnosis. However, disparities remain among racial/ethnic minority populations.
HIV is a neurotrophic organism that enters the CNS soon after exposure (17). HIV causes neuronal injury leading to cognitive impairment. The neurologic manifestations of HIV infection, in the absence of superimposed opportunistic infection or neoplasm, include encephalopathy, myelopathy, peripheral neuropathy, and myopathy.
Pathologically, HIV brain infection causes significant injury. Inflammatory changes habitually occur in the subcortical regions, including the deep white matter and gray matter such as basal ganglia and thalamus. Neurologic dysfunctions are not directly related to neuron infection. A combination of factors, including HIV infection and activation of immunocompetent cells and toxins, may contribute to the pathogenesis of neurologic dysfunction (17). There is an increase in the number of multinucleated giant cells, and macrophages—the cellular target of HIV infection—seem to be related to the progressive encephalopathy seen in AIDS (Fig. 14.14). Polymorphic microglia and, less frequently, astrocytes are also infected. Astrocyte axonal damage has been associated with high viral loads. Myelin pallor and tissue rarefaction is related to the presence of multinucleated giant cells located primarily in the white matter (18).
AIDS dementia complex (ADC), the most common neurologic manifestation in AIDS patients, was previously referred to as subacute encephalitis or subacute encephalopathy. ADC is related to direct viral invasion of neurons (19). Clinical presentation includes progressive dementia with deterioration of mental function associated with movement disorders and/or behavioral dysfunction (34). HIV infection leads to various degrees of neurologic abnormalities that may progress to cognitive impairment and dementia (20).
Clinicopathologic correlation in patients with ADC suggests that the primary pathologic substrate is subacute encephalitis with multinucleated giant cells rather than microglial nodules. Electron microscopy reveals retrovirus particles within these multinucleated giant cells, and their presence strongly correlates with ADC. The multinucleated giant cells may be present within the cortex, basal ganglia, and/or white matter. Early difficulties with memory and concentration are often followed by apparent apathy and social withdrawal and may be mistaken for symptoms of depression. Headache is also a frequent complaint, and seizures are seen in approximately 10% of cases. Diffuse atrophy is usually present. Gross examination often reveals only atrophy.
Initially, lesions are present in the white matter; with disease progression, they can extend to the basal ganglia and cortex. In some patients, the white matter lesions predominate, whereas in others, the gray matter is more severely affected. Lesions may also be located in the brainstem, cerebellum, and spinal cord.
HIV may also directly result in acute encephalitis and acute or chronic meningitis. The viral meningitis typically presents with fever, headache, and meningeal signs, and usually remits spontaneously but can recur. Neuroimaging is usually negative. Clinical symptoms suggest the diagnosis of HIV encephalitis before the evidence of any imaging abnormality. CT is not as sensitive as MR in detecting changes related to HIV infection. In some cases, although MR has shown diffuse white matter disease and HIV has been cultured from brain tissue, the CT has been negative. White matter lesions are infrequently detected by CT in patients with HIV encephalopathy. CT may demonstrate nonspecific diffuse brain atrophy, with no other abnormalities in most subacute encephalitis cases.
Although MRI is more sensitive than CT in depicting brain alterations in HIV-positive individuals, conventional MR sequences are not necessarily able to demonstrate any abnormality in the earliest stages of infection to determine the real extent of damage (17). Cortical atrophy is the most frequent MR finding and is usually the only early modification. A central predominance brain atrophy is noted. Atrophy progresses over time, and some authors correlate this progression with the decline of cognitive abilities. T2-weighted images and FLAIR images reveal hyperintense lesions without mass effect involving much of the periventricular white matter and centrum semiovale that correspond to demyelination and vacuolation (Fig. 14.14). Lesions do not enhance. Lesions may vary from scattered, isolated, unilateral foci to confluent bilateral involvement and may be symmetric or asymmetric (Fig. 14.15). The extent of disease roughly parallels the clinical neurologic deterioration.
The differential diagnosis between HIV encephalitis and progressive multifocal leukoencephalopathy (PML) can be a challenge in clinical and radiologic practices (see subsequent section on PML), but MRI findings form the basis of defining the specific diagnosis in these patients. Although the neuroradiologist
should be able to distinguish these entities in the great majority of cases, variability in size, extent, and distribution of lesions in both HIV-related demyelination and PML, especially early in the course of disease, can preclude a definitive diagnosis based on the MR appearance alone. In general, HIV encephalitis has a pattern of diffuse centrum semiovale and periventricular white matter hyperintensity, in contrast to the unifocal or asymmetric multifocal pattern typically produced by PML (Fig. 14.16). The demyelination lesions of HIV encephalitis tend to be more symmetric and central than those of PML. Although both entities appear hyperintense on T2-weighted images, the lesions of HIV demyelination are usually unapparent on T1-weighted images, whereas those of PML are often clearly hypointense and well-demarcated on T1-weighted images. In PML, lesions often involve the posterior fossa and tend to be located in the posterior portion of the brain. The frontal lobes are the most common sites of involvement in HIV encephalitis. Although neither entity typically has significant contrast enhancement as a major component of its MR findings, it is certainly not uncommon for an edge of enhancement or occasionally tiny foci of enhancement to be seen in PML (Fig. 14.16). Finally, these two entities have different clinical manifestations that are extremely helpful in clinical correlations. HIV encephalitis often manifests as a global encephalopathy, whereas PML is usually associated with a focal neurologic deficit. Given all of these factors, a final confounding issue is that this patient population can simultaneously harbor more than one brain pathology.
should be able to distinguish these entities in the great majority of cases, variability in size, extent, and distribution of lesions in both HIV-related demyelination and PML, especially early in the course of disease, can preclude a definitive diagnosis based on the MR appearance alone. In general, HIV encephalitis has a pattern of diffuse centrum semiovale and periventricular white matter hyperintensity, in contrast to the unifocal or asymmetric multifocal pattern typically produced by PML (Fig. 14.16). The demyelination lesions of HIV encephalitis tend to be more symmetric and central than those of PML. Although both entities appear hyperintense on T2-weighted images, the lesions of HIV demyelination are usually unapparent on T1-weighted images, whereas those of PML are often clearly hypointense and well-demarcated on T1-weighted images. In PML, lesions often involve the posterior fossa and tend to be located in the posterior portion of the brain. The frontal lobes are the most common sites of involvement in HIV encephalitis. Although neither entity typically has significant contrast enhancement as a major component of its MR findings, it is certainly not uncommon for an edge of enhancement or occasionally tiny foci of enhancement to be seen in PML (Fig. 14.16). Finally, these two entities have different clinical manifestations that are extremely helpful in clinical correlations. HIV encephalitis often manifests as a global encephalopathy, whereas PML is usually associated with a focal neurologic deficit. Given all of these factors, a final confounding issue is that this patient population can simultaneously harbor more than one brain pathology.
Some evidence supports the idea that the CNS is a reservoir for HIV. A viral load increase is associated with a decline in neurocognitive functions. Early in the course of infection, HIV enters the CNS (21). Among asymptomatic HIV-seropositive patients, brain atrophy and multifocal signal abnormalities on T2-weighted images have been shown. MR abnormalities have been reported in approximately 50% of patients with an AIDS-related complex and around 70% of patients with AIDS. These abnormalities included sulcal and ventricular enlargement and
patchy areas of hyperintensity on T2-weighted images without mass effect. Over time, in parallel with the clinical progression of cognitive deterioration, white matter lesions become more diffuse, homogeneous, and confluent, and cerebral atrophy increases (22).
patchy areas of hyperintensity on T2-weighted images without mass effect. Over time, in parallel with the clinical progression of cognitive deterioration, white matter lesions become more diffuse, homogeneous, and confluent, and cerebral atrophy increases (22).
In a follow-up study, MR tests were performed in asymptomatic HIV-seropositive patients and then repeated 2 to 4 years later. Eighty percent of these tests were found to be normal and remain normal. Twenty percent had minor abnormalities that were static and stable. Serial examinations of patients with mild neurologic symptoms revealed that 50% of MR scans were mildly abnormal but remained stable. The MR findings of minor abnormalities in asymptomatic HIV-seropositive patients may not be clinically significant because they apparently remain stable.
HAART is a treatment regimen that causes suppression of viral replication, resulting in a decreased viral load (often to undetectable levels). This is essential for neurologic integrity because high CSF viral loads clearly correlate with cognitive impairment in patients with HIV-related dementia. Other effects of this treatment include increased CD4 counts, as well as decreased morbidity and increased survival (17).
Drug treatments with zidovudine and HAART (21) have shown reductions in CSF viral loads, reductions in white matter HIV-related lesions on MRI, and improvements in cognitive function. A neuroimaging marker for HIV-associated neurofunctional changes in the brain is essential for detecting early modifications in mental status. Thus, these techniques could be useful in measuring response to HAART (21).
Proton MR spectroscopy is a valuable tool in the assessment of patients with HIV infection. This MR method is able to depict brain metabolite changes in asymptomatic HIV-infected patients with no abnormalities on conventional MRI sequences. Thus, it seems that proton MR spectroscopy may reveal cerebral abnormalities before they are apparent on standard imaging and holds a promise of early diagnosis of biochemical alterations in HIV-infected patients.
A reduction in the levels of NAA (Fig. 14.17), a neuronal functional marker, is evident in the early onset of infection, even in asymptomatic individuals. The decline is greater in symptomatic than asymptomatic patients. The reduced N-acetylaspartate/creatine (NAA/Cr) and N-acetylaspartate/choline (NAA/Cho) ratios support a theory of neuronal loss and/or dysfunction as a contributing factor to ADC and seem to indicate subtle biochemical modifications that correlate with the clinical neurologic findings but were completely unapparent on conventional imaging studies. However, some patient may have abnormal imaging and a normal spectrum. Thus, the information provided by the two techniques appears to be complementary (Fig. 14.18). An eventual drop in NAA/Cr ratio appears to correlate with the progressive neurologic deterioration in patients with more advanced disease. Whole-brain NAA measurement has also demonstrated a diffuse reduction both in symptomatic and asymptomatic patients before abnormalities in parenchymal brain signal intensity.
Some authors have found that although NAA/Cr and NAA/Cho ratios are reduced in patients with moderate to severe ADC, patients with early ADC or no dementia have MR spectra similar to those of control subjects (23). NAA levels may improve in some patients with ADC in response to treatment.
Choline is a cell membrane metabolite marker that may represent increased cell membrane turnover and/or membrane disruption with cellular damage. MRS may reveal an increase in choline compounds earlier than the NAA reduction in patients with cerebral HIV infection (24). Thus, an elevated choline level appears to be a useful marker for early cerebral injury secondary to HIV and is high before the onset of clinical dementia (24). The increased choline is present in both early and late stages of HIV infection. Choline levels are not directly related to clinical status or CD4 counts.
mI is a marker for neuroglial cell activation. Atrophy and gliosis may lead to an increase of mI as a result of neuroglial cell proliferation with replacement of damaged neurons. A high mI/Cr ratio has been seen in patients with atrophy and diffuse white matter disease on MR, as well as in HIV patients without dementia. Some investigators have found a less prominent elevation in mI/Cr in patients with ADC. Although mI seems to be elevated in the early course of cerebral HIV infection, it may be less prominent with advancing dementia (25). In summary, MRS analysis in early stages of HIV dementia seems to be associated with an increase in both choline and mI levels.
On MRS examination after use of HAART, a normalization of the Cho/Cr ratio in the basal ganglia and a reduction of the mI/Cr ratio in the basal ganglia and frontal lobe white matter were noted. This decline correlated well with changes in CD4 counts and improvement in ADC stage. The authors suggested that mI might be a very good in vivo marker for monitoring brain injury in patients with HIV infection. The early stages of HIV dementia may be treatable and possibly reversible.
Diffusion imaging can show white matter abnormalities even though no structural changes are seen on conventional MRI. This finding may be consistent with early MRS metabolite abnormalities in the white matter. Among HIV patients with dementia, white matter hyperintensity on T2-weighted imaging and FLAIR imaging is frequently observed. A significant increase in diffusivity and reduction in fractional anisotropy (FA) values may be related to an increase in viral load (21,26). These changes have been noted prior to the onset of cognitive impairment. Whole-brain diffusion tensor imaging (DTI) correlates with the severity of dementia. Thus, DWI and DTI sequences may be important for the early detection and initiation of ADC treatment. The disruption of white matter fiber microstructure integrity may provide an early indication of the potential for HIV-associated dementia (Fig. 14.19) (18). Although these findings seem promising, a previous report did not demonstrate significant usefulness of DTI in depicting early diffusion abnormalities of normal-appearing white matter in patients with HIV infection (17).
Immune Reconstitution Inflammatory Syndrome (IRIS)
The immune reconstitution inflammatory syndrome (IRIS) occurs most commonly in the setting of HIV immunosuppression. It is defined as a paradoxic deterioration of clinical response encountered in HIV-infected patients who have received HAART weeks, months, or rarely years ago. The median time for onset of IRIS occurs within 60 days after the institution of HAART (27). IRIS represents an exuberant inflammatory response to an antigen that is either to a dead or dying organism resulting from an opportunist infection or a viable pathogen from a persistent infection, or to self-antigens (28).
Although this robust inflammatory response is usually self-limiting, it has been reported an overall incidence as high as 25% to 35%, increasing to 45% in those patients with underlying opportunistic infections (27). The diagnosis is often challenging, treatment options are limited, and the prognosis is variable. IRIS is a diagnosis of exclusion. Neuroradiologists play an essential role in the assessment of this condition, and to predict the diagnosis. Some imaging clues can be depicted to help its correct identification, such as contrast enhancement, transient increase in parenchymal abnormalities with high signal on FLAIR, mass effect, and restricted diffusion (Fig. 14.20). Pathologically, there is a T-cell infiltration, as a result of an intense cellular proliferative response. It is essential to recognize this condition so that appropriate therapy can be initiated.
Although this robust inflammatory response is usually self-limiting, it has been reported an overall incidence as high as 25% to 35%, increasing to 45% in those patients with underlying opportunistic infections (27). The diagnosis is often challenging, treatment options are limited, and the prognosis is variable. IRIS is a diagnosis of exclusion. Neuroradiologists play an essential role in the assessment of this condition, and to predict the diagnosis. Some imaging clues can be depicted to help its correct identification, such as contrast enhancement, transient increase in parenchymal abnormalities with high signal on FLAIR, mass effect, and restricted diffusion (Fig. 14.20). Pathologically, there is a T-cell infiltration, as a result of an intense cellular proliferative response. It is essential to recognize this condition so that appropriate therapy can be initiated.
Some risk factors for developing IRIS can be listed as follows: HAART-naïve patient; severely immunocompromised with very low CD4 counts (<50 cells/mm3); high HIV viral load; after HAART initiation, a rapid and prominent drop in HIV viral load and rising CD4 counts; opportunistic infection when HAART is initiated; resumption of therapy after an interruption. In those HIV-infected patients, the immune system is reconstituting.
IRIS can affect any organ in the body, such as lungs, liver, and lymph nodes. The incidence of CNS-IRIS ranges from 0.9% to 1.5%. Clinically, the patients may develop recurrence of the initial symptoms associated with their infection or they may develop new inflammatory symptoms following institution of HAART, such as fever, painful enlarged lymph nodes, and headache (27). “Delayed” or “paradoxical” IRIS is defined to the inflammatory reaction to a persistent antigen. When the robust inflammatory response is a reaction to a viable pathogen related to a latent infection, the term “unmasking” or “simultaneous” IRIS is used (27). Some pathogens are more likely to be associated with CNS-IRIS, such as PML, VZV, CMV, HIV encephalitis, cryptococcal meningitis, mycobacterial infections, and toxoplasma encephalitis (Fig. 14.21).
PML-IRIS accounts for as many as 18% of the HIV-infected patients with PML. The use of early and prolonged steroids has been suggested as an efficient treatment.
Previous reports demonstrated that patients with higher mI levels at MRS analysis and the presence of lesional contrast enhancement were pointed out as being another positive predictor value for increased survival (29). A transient increase in high FLAIR signal and contrast enhancement in the white matter, which is seen in only 56% of patients, and subsequent leukomalacia and atrophy also correlate with increased survival (29). DWI can also be useful to predict outcome in some patients. Peripheric area of restricted diffusion has been seen in
PML lesions. The ADC values of PML lesions were different for rapid versus slow clinical progression after HAART. Lesions with higher maximum ADC ratios and high John Cunningham virus (JCV) titers before initiation of HAART therapy is told to be more likely to progress rapidly and may be considered a risk factor for IRIS. However, lower ADC values were associated with stable lesions or remyelination.
PML lesions. The ADC values of PML lesions were different for rapid versus slow clinical progression after HAART. Lesions with higher maximum ADC ratios and high John Cunningham virus (JCV) titers before initiation of HAART therapy is told to be more likely to progress rapidly and may be considered a risk factor for IRIS. However, lower ADC values were associated with stable lesions or remyelination.
Cryptococcal-IRIS can be manifested in many different ways, such as lymphadenitis, pneumonitis, meningitis, or cryptococcomas, with a reported mortality ranging up to 30%. Clinically patients develop headache, fever, malaise, altered mental status, raised intracranial pressure, and cranial nerve palsies in the setting of lymphadenopathy and new pulmonary infiltrates. Neuroimaging aspects differ between cryptococcal-IRIS (CM-IRIS) and HIV-related cryptococcosis. In CM-IRIS, patients may present an intense leptomeningeal enhancement, communicating hydrocephalus, linear perivascular enhancement in the sulci, and new meningeal or choroid plexus enhancement. Enhancement of the vascular spaces is a characteristic of CM-IRIS. Secondary involvement of brain parenchyma may be demonstrated as areas of hypersignal intensity on FLAIR/T2-weighted images, restricted diffusion, and parenchymal enhancement (28).
Pediatric Human Immunodeficiency Virus
According to the World Health Organization, 3.2 million children are living with HIV/AIDS worldwide. Although between 2002 and 2013, there was a 58% reduction in the number of new HIV infections among children, in 2013, 240,000 were newly infected and 190,000 died of AIDS-related illnesses, out of 1.5 million people overall. In the United States, approximately 2% of AIDS patients are children, and 5% to 25% of
AIDS cases worldwide occur in children. More than 90% of children living with HIV live in sub-Saharan Africa. Around 90% of pediatric AIDS is secondary to congenital infection via maternal-to-child transmission (vertical acquisition), during pregnancy, labor, delivery, or breastfeeding.
AIDS cases worldwide occur in children. More than 90% of children living with HIV live in sub-Saharan Africa. Around 90% of pediatric AIDS is secondary to congenital infection via maternal-to-child transmission (vertical acquisition), during pregnancy, labor, delivery, or breastfeeding.
The prevalence of CNS disease in HIV-infected children ranges about 20% to 60%, and the onset of neurologic disease generally occurs between 2 months and 5 years of age (30). The neurologic complications of AIDS are HIV encephalopathy and opportunistic CNS infections.
Pediatric HIV infection may result in an encephalopathy with cognitive and behavioral features, including motor impairment and the development of weakness and pyramidal tract signs (31). HIV encephalopathy is divided into progressive and static encephalopathies. This progressive encephalopathy is estimated to occur in 30% to 50% of HIV-seropositive children and is comparable to ADC. In static encephalopathy, children have better brain function, but have delay in the age-appropriate milestones (31). The subacute encephalitis is likely due directly to the HIV infection. The most frequent symptoms and signs of HIV infection are cognitive impairment, delayed mental development, microcephaly, abnormalities of motor function and tone, seizures, and ataxia.
Brain atrophy is the most common imaging finding and has been reported to occur in approximately 57% to 86% of HIV-infected children. Both CT and MRI modalities can easily show atrophy. Three different patterns of atrophy have been described: necrotizing encephalopathy with encephalomalacia; generalized atrophy, particularly affecting the frontal lobes; and central atrophy, due to preferential tropism of the virus for the basal ganglia and deep white matter causing necrosis and atrophy with disproportionate ventriculomegaly, as compared with cortical atrophy (31). Generalized cerebral parenchymal atrophy is less commonly observed and is more frequent in symptomatic children (Fig. 14.22). Children presenting with progressive encephalopathy, brain atrophy, and neurologic symptoms may experience symptom reversal with antiretroviral therapy.
Up to 44% of HIV-infected children present with white matter lesions on imaging analysis. These lesions are most often seen in the late stages of infection. MRI is superior to CT in depicting white matter disease. Patchy areas of white matter hyperintensity on T2-weighted images are evident and may become confluent with disease progression. The lesions tend to involve the periventricular white matter and centrum semiovale. The progression of white matter involvement correlates well with the progression of dementia. T2 signal hyperintensity may be seen in the basal ganglia of some patients. Due to the unmyelinated white matter during the first 18 months, appreciation of these abnormalities may be difficult. Usually, no mass effect or contrast enhancement is observed. Differently to PML, these lesions tend to be more bilateral, symmetric, and diffuse.
Basal ganglia calcification may be seen without inflammatory disease and with no sign of acute infection. Reduced brain volume is likely related to myelin loss or diminished myelination because neuronal loss is uncommon.
Calcification can be detected in about one-third of HIV-infected children and is often present in the globus pallidus and putamen, and is usually bilateral. Thus, CT can suggest this entity with more accuracy than MR, an imaging technique with low sensitivity to calcification. Less frequently, white matter of the frontal lobes may present calcifications. Enhancement of the basal ganglia has also been reported. On MR, basal ganglia calcifications are often invisible but can be seen as hypointense areas on T2-weighted images and susceptibility-weighted images, or even hyperintensity on T1-weighted images. This is a unique finding related to vertical transmission. Calcifications related to HIV infection are usually not seen before 10 months of age. Initial detection occurs by the age of 1 to 5 years, and the disease generally presents a progressive course, becoming more evident over time.
MRS has revealed reduced NAA/Cr ratios in the subcortical structures of children with AIDS and a more significant decrease in those children with AIDS encephalopathy (57).
Although CMV, toxoplasmosis, and PML have all been described in pediatric AIDS patients, opportunistic infections are far less common in children than in HIV-infected adults (58). This is partly due to a lack of exposure because many of these infections result from reactivation. Approximately 15% of neuroimaging studies in pediatric AIDS patients reveal a focal lesion as a result of an opportunistic infection or CNS lymphoma.
CNS lymphoma is the most common intracranial mass lesion in pediatric AIDS patients and occurs in up to 4% of cases. Age at presentation ranges from 5 months to 10 years. These lesions show edema and mass effect, as well as enhancement. Lymphomas may be solid, heterogeneous, or ring enhancing as a result of central necrosis. Lesions are usually multicentric, and there may be subependymal spread.
There is a 1.3% annual risk for developing cerebrovascular disease in HIV-infected pediatric population (32). Ischemic and hemorrhagic strokes are commonly seen in children with AIDS. Arterial ectasia characterized by a fusiform, aneurysmal dilation of a branch of the circle of Willis may be seen as an isolated finding or may be found in conjunction with ischemia or hemorrhage (Fig. 14.23). The enlarged ectatic vessels are well demonstrated on contrast CT studies. MRI reveals a signal void within the ectatic vessels, mainly on T2-weighted MR images. Magnetic resonance angiography (MRA) is helpful in delineating the extent of involvement. Histopathologic examination of these enlarged vessels reveals vasculitis with infected mononuclear cells throughout the arterial wall, destruction of the elastic lamina, and subintimal fibrosis.
Fibrosing sclerosis is an inflammatory fibrosing vasculopathy that has also been reported in children with HIV infection. This sclerosis is probably a direct result of CNS infection by HIV. Single or multiple ischemic infarctions may result and may have a slight mass effect. MRA may reveal focal vascular regions of stenosis or occlusion. Histopathologic evaluation
of these diseased vessels reveals endothelial proliferation with luminal obliteration or stenosis, as well as damage to the elastic lamina, thickening of the media, and inflammation of both the media and the adventitia.
of these diseased vessels reveals endothelial proliferation with luminal obliteration or stenosis, as well as damage to the elastic lamina, thickening of the media, and inflammation of both the media and the adventitia.
JC Polyomavirus—Progressive Multifocal Leukoencephalopathy
PML is a rare opportunistic subacute progressive demyelinating disorder caused by JC polyomavirus in immunocompromised patients. The histopathologic hallmark of PML is demyelination with enlarged oligodendroglial nuclei and bizarre astrocytes (32).
The JC virus is widespread, with antibodies found in 50% to 70% of the human population by the age of 65 years. The infection does not produce overt illness and remains dormant in the kidneys, tonsils, possibly B lymphocytes, and bone marrow unless there is reactivation due to immunodeficiency (33) or immune suppression. AIDS and other immunodeficiency conditions, including lymphoproliferative and myeloproliferative disorders, chronic granulomatous diseases, sarcoidosis, and use of immunosuppressive drugs for organ transplantation, rheumatologic conditions and mAb therapy, like natalizumab, are associated with PML. Although PML is associated to severe depletion of cellular immunity, recently reports have associated this condition with less overt immunodeficiency, such as cirrhosis, renal failure, psoriasis, dermatomyositis, and even pregnancy (34). The prevalence of PML has increased greatly since the beginning of the AIDS epidemic. It appears to have a stronger association with AIDS because the incidence of PML increases concomitantly with the rise in AIDS. In approximately 0.7% to 11% of patients who have HIV develop PML during the course of the illness, and recently, up to 85% of PML cases have been attributable to AIDS. Since the introduction of HAART, the incidence of PML has shown a tendency to diminish. Clinical and imaging-documented improvements, associated with longer survival, have also been reported.
Clinically, PML is characterized by progressive neurologic deficits leading to death (without therapy) in approximately 2.5 to 4 months of the onset of symptoms (32). PML causes death in 90% of patients within 1 year after diagnosis. Only a small number of cases have a more benign clinical course. The most common symptoms of PML are hemiparesis, visual impairment, and altered mentation, whereas patients with lesions of the posterior fossa exhibit ataxia, dysarthria, and dysmetria. Spinal cord involvement is rare. Approximately 20% of patients develop seizures in addition to the focal neurologic symptoms. Other clinical features include memory loss, visual deficit (frequently homonymous hemianopsia), personality changes, cognitive and speech disturbances, altered mental status, and motor and/or sensory abnormalities with a progressive neurologic decline. Less frequent signs and symptoms include vertigo, seizures, headache, and aphasia.
Brain biopsy is the most definitive way to establish a specific diagnosis of PML, in which the triad of demyelination, giant atypical astrocytes, and oligodendrocytes with intranuclear inclusion bodies is found (Figs. 14.16 and 14.24). New imaging tools and CSF studies are emerging as potential mechanisms to replace brain biopsies in the diagnosis of PML (33). The
need for a less invasive method has been recognized. However, most CSF analyses using PCR testing are normal (35). Sensitivity and specificity for PML detection in the CSF via PCR are about 92%. A positive CSF PCR and a high JC virus DNA load are associated with diminished survival.
need for a less invasive method has been recognized. However, most CSF analyses using PCR testing are normal (35). Sensitivity and specificity for PML detection in the CSF via PCR are about 92%. A positive CSF PCR and a high JC virus DNA load are associated with diminished survival.
PML appears as multifocal hypodense white matter lesions involving the subcortical or periventricular regions without causing significant mass effect and usually without enhancement on imaging. CT, however, is frequently negative, especially in early stages of disease, so it is virtually never used to image these patients unless MRI is unavailable. Patchy enhancement at the periphery of lesions is seen in less than 10% of cases. As in any modality, serial scans indicate progressive disease.
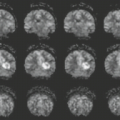
MR demonstrates far greater sensitivity than CT in the imaging of PML for defining both the extent and the number of lesions and is always the procedure of choice in imaging any patient with suspected PML. PML lesions on MR images are hypointense on T1-weighted images and hyperintense on T2-weighted images involving the periventricular and/or subcortical white matter, producing a “scalloped” appearance (Figs. 14.25 to 14.27). Initially, lesions may be small, but they usually progress to larger areas of involvement (Figs. 14.28 and 14.29). Lesions usually have a bilateral, multifocal, and asymmetric distribution pattern, and may occur in any location in the white matter, most often in the parietal lobe, followed by the frontal lobe (32) (Fig. 14.27). Mass effect is infrequent and mostly minimum. PML lesions are estimated to enhance in about 10% of cases on MR. When enhancement is present, it is faint and typically at the periphery of lesions In some cases, there may be microcysts at the center of an active lesion on T2-weighted image (Fig. 14.20) (36). It is believed to signify a more intense inflammatory reaction and may be a predictive factor for prolonged survival (17); however, it has also been reported in aggressive cases associated with certain pharmacologic treatments of multiple sclerosis (MS) (Fig. 14.30) (37). Occasionally, mild mass effect is present (35). It has been suggested that mass effect, increased atrophy, confluence of lesions, and increased hypointensity on follow-up T1-weighted MR studies can be regarded as indicators of a poor prognosis in untreated AIDS patients with PML. However, only the mass effect was related to predicting the risk of death in patients with PML. PML may also appear to affect the deep gray structures due to involvement of small myelinated fibers that course through the basal ganglia and adjacent structures (Fig. 14.26). The thalamus is the most common affected region, followed by the basal ganglia. Usually the gray matter lesion is associated with white matter involvement.
PML commonly affects the posterior fossa (Fig. 14.16). Approximately one-third of PML cases have some involvement of the posterior fossa. There are often synchronous with supratentorial lesions, although PML may be limited to the infratentorial structures in about 10% of cases. Typically the lesions involve the middle cerebellar peduncle and adjacent pons and cerebellum.
In AIDS patients, PML lesions do not typically resemble HIV encephalopathy causing demyelination, but they are occasionally difficult to distinguish on imaging (see the earlier section on HIV encephalopathy). As stated earlier in more detail, PML is more often discrete, multifocal, and asymmetric, with scalloping and a greater predilection for the subcortical white matter. HIV encephalitis is more often ill-defined, diffuse, symmetric, and periventricular in location. Clinical correlation is
important as well, as mentioned earlier, because HIV encephalitis most often presents with global cognitive disturbances and dementia, whereas PML presents with a focal motor or sensory deficit.
important as well, as mentioned earlier, because HIV encephalitis most often presents with global cognitive disturbances and dementia, whereas PML presents with a focal motor or sensory deficit.
Some new MR sequences may play a role in detecting subtle abnormalities in PML patients, such as MT, DWI, DTI, and MRS (33). In the research literature, MT MRI seems to be more sensitive for depicting demyelination processes than conventional imaging in group studies. Thus, it may be possible indirectly to verify the integrity of the myelin sheaths. It may also be feasible, according to a previous report, to distinguish the destructive demyelination of PML from the edematous changes related to HIV encephalopathy. Quantitative MT methodology, however, has not found its way into clinical imaging protocols on individual patients at the time of this writing.
MRS has also been used to characterize and differentiate PML from other CNS HIV-related processes (Fig. 14.31). NAA reductions and creatine peaks have been seen (38). There is also an increase in choline, excess lipids, and lactate, as well as an occasional increase in mI. These changes seem to
parallel some of the pathologic features of PML. Decreased NAA shows an association with neuronal loss, cell membrane and myelin breakdown cause an increase in Cho, and a rise in glial activity leads to increased mI (38). A marked elevation of mI/Cr levels in the early stage of the disease may be related to a favorable outcome. The extent of the inflammatory reaction is directly related to the high mI peak elevation. Early in the course of PML, elevations in Cho and mI are seen, secondary to the inflammatory reaction and myelin destruction. In more advanced disease, neuronal compromise leads to a progressive decrease in NAA and an eventual decrease in all metabolites. The mild elevations of lactate and lipids likely result from macrophages and the breakdown of cell membrane and myelin (38).
parallel some of the pathologic features of PML. Decreased NAA shows an association with neuronal loss, cell membrane and myelin breakdown cause an increase in Cho, and a rise in glial activity leads to increased mI (38). A marked elevation of mI/Cr levels in the early stage of the disease may be related to a favorable outcome. The extent of the inflammatory reaction is directly related to the high mI peak elevation. Early in the course of PML, elevations in Cho and mI are seen, secondary to the inflammatory reaction and myelin destruction. In more advanced disease, neuronal compromise leads to a progressive decrease in NAA and an eventual decrease in all metabolites. The mild elevations of lactate and lipids likely result from macrophages and the breakdown of cell membrane and myelin (38).
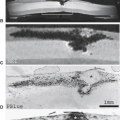
DWI can be used to monitor the degree of demyelination (39). The appearance of PML lesions on DWI varies according to the disease stage. DWI may show increased apparent diffusion coefficient values in the central area of the lesion, surrounded by a small rim of decreased diffusibility. This may represent a heterogeneous pathologic process in which the central area corresponds to complete tissue injury with necrosis and reduced cellularity. The peripheral region consists of active tissue injury (Figs. 14.32 and 14.33). The presence of multiple small areas of decreased diffusion throughout the entire cerebral white matter may indicate a merging of multiple areas of tissue injury to form large confluent lesions (39). DTI shows a reduction of FA values when apparent diffusion coefficient values are normal and no abnormality is demonstrated on T2-weighted images (40) (Fig. 14.34). The reduction of FA values follows the pathologic sequence of PML events. In an early phase, these reduced FA values represent an injury of myelin sheaths. It is followed by secondary diffuse cellular loss, leading to increased apparent diffusion coefficient values. DTI and DWI may be of great use in the management of PML patients for guiding and monitoring the treatment (39,40).
Besides brain biopsy, diagnosis of PML can be established by MRI or demonstration of JCV DNA by PCR of the CSF (36). However, it has become frequent to have a negative CSF exam in AIDS patients with PML. Recently, the diagnostic criteria classify PML as “definite PML” or “presumptive PML.” “Definite PML” is considered when there are consistent clinical and imaging findings associated with a positive PCR or defined in the brain biopsy. In the “presumptive PML,” clinical and imaging findings are not associated with a positive JCV DNA in the CSF.
No established treatment has been approved for PML. Recently, various drugs have been used that have shown some improvement in patient survival. The widespread introduction of HAART has led to a sharp decline in both morbidity and mortality associated with opportunistic infections. PML patients treated with HAART display reduced CSF detection of the JC virus, increased CSF production of antibodies to JCV-VP-1, increased survival, improved neurologic status, and improved or stabilized MR findings after 12 months of treatment. Survival improvement after HAART has been observed in some PML patients but not in all.
With restoration of immunity with HAART, there may be an abrupt change in the clinical behavior and histopathologic and imaging manifestations of PML in some patients (36). This altered presentation has a profound clinical significance and needs to be differentiated from the classic presentation. Three new CNS manifestations of JC virus infection have been recently recognized and described: meningitis (JCM), encephalopathy (JCE), and granular cell neuronopathy (JVCGCN).
There is a characteristically involvement of the cerebellum in the JVCGCN, which infects only cerebellar granule cell neurons, sparing the oligodendrocytes. Clinical symptoms are restricted to the cerebellar involvement, including ataxia and dysarthria. Some reports demonstrated JCV infections causing meningoencephalitis in immunocompetent patients (36). More recently, an encephalopathic form of CNS infection by JCV was described.
PML is typically characterized by a distinct lack of inflammatory change. Rarely, reactivation of the JCV and development of PML can be associated with a marked inflammatory reaction (36). A paradoxical worsening of inflammatory changes is noted in the first few months after the onset of HAART (33). A marked enhancement may be seen soon after the beginning of HAART, corresponding to an increased inflammatory response, attributable to the immune reconstitutive effect, associated with lesions associated with mass effect and vasogenic edema (17). In patients with PML, the risk of death is decreased by as much as 60% if the treatment regimen includes a protease inhibitor. However, HAART does not prevent progression of PML in all cases and may not be effective in all patients who develop PML while receiving HAART. Approximately half of the patients who have AIDS with PML do not experience benefit from HAART (Table 14.1) (32).
More recently, patients with MS that underwent to monoclonal antibody therapy using natalizumab developed PML (Fig. 14.28) (37). One possible explanation is a natalizumab-triggered reactivation of latent JC virus.
Human T-Cell Lymphotropic Virus Type 1
Human T-cell lymphotropic virus type 1 (HTLV-1) infection is extremely rare in developed countries. In the United States, approximately 46,000 persons are infected with HTLV-1. However, HTLV-1 is endemic in developing countries. HTLV-1 can cause tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) and adult T-cell leukemia/lymphoma. HAM and TSP are characterized by a slowly progressive disabling disorder, with paraparesis, mild sensory disturbance, and urinary incontinence. Other conditions, such as isolated peripheral polyneuropathy, myopathy, arthropathy, and uveitis, have been associated with HTLV-1. Apparently, HTLV-1 causes disease in a minority of the infected population, with prevalence of neurologic involvement less than 3%.
TABLE 14.1 Differential Diagnosis: Human Immunodeficiency Virus (HIV) Versus Progressive Multifocal Leukoencephalopathy (PML) | |||
|---|---|---|---|
|
Although clinically similar to certain forms of MS, TSP is a distinct entity based on epidemiologic studies and serologic tests. The white matter lesions of TSP/HAM are in some aspects similar to those observed in patients with MS or in HIV-infected people. Chronic perivascular inflammatory changes occur diffusively in the CNS, leading to multifocal leukoencephalopathy.
Autopsy studies have shown that both HAM and TSP involve primarily the spinal cord, especially the thoracic cord, where inflammatory changes, demyelination, and axonal loss may be seen. A more widespread chronic meningoencephalomyelitis is also present, with inflammatory changes involving the meninges, brainstem, and both supratentorial and infratentorial white matter. Thickening of the media and adventitia of blood vessels in the subarachnoid space, the spinal cord, and in the brain suggests vasculitis as well. Although HTLV-1 has been isolated from CSF lymphocytes of a patient with HAM, it is uncertain whether the neuropathologic changes are due to HTLV-1-infected lymphocytes or direct neurotropism of the retrovirus.
MRI reveals punctate and nodular hyperintense white matter lesions on T2-weighted imaging and FLAIR imaging in the periventricular and subcortical white matter in more than 60% of HTLV-1-infected patients (41) (Fig. 14.35). The white matter lesions do not enhance or cause mass effect. Lesions are most often discrete but may be confluent. The appearance is nonspecific and must be differentiated from other white matter lesions such as MS, Lyme disease, HIV-related demyelination, ischemia, and vasculitis. Serial studies reveal an increase in the number of these white matter lesions in affected patients. The spinal cord can also be involved, presenting foci or multifoci hyperintense lesions.
TABLE 14.2 Human Prion Diseases | |||||
|---|---|---|---|---|---|
|
Human Prion Diseases
Prion diseases, also known as transmissible spongiform encephalopathy, comprise a group of very rare progressive neurodegenerative disorders that affect both humans and nonhuman animals. These diseases are recognized on pathology by their characteristic spongiform brain changes with neuronal loss and a lack of inflammatory response and are clinically known for long incubation periods (32).
The transmissible agent of these disorders is believed to an abnormal form of human prion protein. Normal-occurring human prion protein is coded by a gene on the short arm of chromosome 20. The normal prion protein, called cellular prion protein (PrPc) is rapidly degraded by proteolysis, whereas the abnormal form, termed scrapie prion protein (PrPsc), is not. The agent induces abnormal folding of normal cellular prion proteins in the brain, leading to accumulation of PrPsc in the brain in plaques. Brain damage and the characteristic signs and symptoms of the disease ensue. Prion diseases are usually rapidly progressive and always fatal. A number of different specific mutations of the PrP have been identified in familial cases (Table 14.2).
Human prion disease had traditionally been classified into three clinical syndromes: kuru, Creutzfeldt–Jakob disease (CJD), and Gerstmann–Straüssler–Scheinker (GSS) disease. However, with recent advances in understanding the etiology of prion disease, a more appropriate way to consider CJD is to divide it into familial (inherited), sporadic, and acquired subtypes. The sporadic forms are divided into sporadic Creutzfeldt–Jakob disease (sCJD) and sporadic fatal insomnia. The familial prion diseases
consist of familial CJD, fatal familial insomnias, and GSS disease. The acquired diseases are kuru, iatrogenic CJD, and new variant CJD (vCJD) (42).
consist of familial CJD, fatal familial insomnias, and GSS disease. The acquired diseases are kuru, iatrogenic CJD, and new variant CJD (vCJD) (42).
Kuru is a neurodegenerative disorder characterized primarily by cerebellar ataxia. Kuru was quite common in the first half of the twentieth century in members of the Fore linguistic group living in the eastern highlands of Papua New Guinea. Transmission was from ritual cannibalism. Because this practice was outlawed in the 1950s, kuru is now almost nonexistent. Kuru is clinically characterized by progressive cerebellar ataxia, and, in contrast to CJD, dementia is not a prominent feature.
GSS originally was described as an inherited condition characterized primarily by cerebellar ataxia, with dementia occurring late in the disease. In contrast to CJD, the clinical course in GSS is more prolonged, with a mean duration of 5 years and clinical onset in the 20s to 30s.
Creutzfeldt–Jakob Disease
The human prion disease syndrome most often associated with dementia is CJD. The annual incidence is estimated at 0.25 to 2 cases per million population. Peak incidence in the naturally acquired disease occurs between 60 and 64 years of age (43). Like all forms of human prion disease, CJD is uniformly fatal. Although quite rare, it is an extremely important diagnosis to ascertain because it has major implications for public policy and epidemiology concerns (74).
CJD can be divided into the following four clinical forms: sporadic, familial (inherited), variant, and iatrogenic. The sporadic form is the most common and accounts for about 85% of the cases, whereas 10% to 15% of cases are familial. A few cases have been linked to the iatrogenic form, and most of them have been associated with corneal transplants, injection of human growth hormone, cortical electrode implantation, and dura mater grafts. vCJD is acquired by eating contaminated meat from cows infected with bovine spongiform encephalopathy or, more commonly, “mad cow” disease.
The vCJD is seen in younger individuals, whereas the sporadic form is more common among elderly patients. The classic clinical diagnostic triad, described as rapidly progressive dementia, myoclonic jerks, and periodic sharp-wave EEG tracing, may be lacking in up to 25% of patients. Some patients present with sensory abnormalities, confusion and/or inappropriate behavior, or cerebellar ataxia. Prognosis is extremely poor, with a mean survival of less than 1 year from the onset of symptoms and only 10% of patients surviving for more than 2 years. Development of symptoms occurs over weeks, and 70% of affected patients die within 6 months. EEG typically reveals diffuse slowing with superimposed bursts of sharp waves. CSF is often normal but may show a slightly elevated protein content. The variant form has a prominent psychiatric feature early in the disease and a slower progressive course, with a median survival of 14 months. In contrast to sporadic CJD, the periodic EEG and the characteristic CSF analysis is not as sensitive to the diagnosis of vCJD and cannot discriminate among the various forms of the disease. In these patients, MRI findings have an important role in the correct diagnosis.
Pathology in CJD is characterized by spongiform change, neuron loss, and astrocytosis (Fig. 14.36). Inflammatory changes are absent. CJD can be divided into three main pathologic variants: frontoparietal (33%), occipitoparietal (18%), and diffuse cortical/basal ganglial (49%) forms. Any of these variants may also reveal lesions of the thalamus, midbrain, cerebellum, or spine.
As already stated, definitive diagnosis of CJD can only be made with histopathologic examination, but MRI is so specific that some use imaging as proof of diagnosis in conjunction with clinical and EEG findings. The diagnosis of probable CJD is made in a patient with characteristic neuropathology, typical EEG alterations (periodic epileptiform discharges), or detection of 14–3–3 protein in the CSF. A diagnosis of possible CJD can be considered when no typical EEG changes are found. Nonetheless, EEG alterations are not always specific for CJD because they have been described in other neurologic conditions, such as Alzheimer disease, cerebral ischemia, and frequent seizures. Sensitivity may vary from 65% to 85%, especially in the late phase of the disease.
 FIGURE 14.36 Creutzfeldt–Jakob disease (CJD). Spongiform change is demonstrated in this hematoxylin-eosin–stained specimen from a patient with autopsy-proven CJD. |
MR is highly sensitive and specific for the detection of CJD abnormalities, and many believe that it has essentially become the de facto proof of diagnosis. Although hypodense lesions of the occipital lobes and basal ganglia have been reported, CT is not an appropriate imaging study in these patients other than to initiate a workup to exclude unrelated focal pathology that may contribute to similar clinical symptoms. MR typically demonstrates a symmetric T2 hyperintensity involving the basal ganglia, particularly the striatum, caudate nuclei, putamen, thalamus, and periventricular white matter. Cortex abnormality, symmetric or asymmetric, can be observed in the absence of striatal involvement (Figs. 14.37 and 14.38). Lesions do not enhance and do not demonstrate mass effect, in contrast to other encephalitides with more acute symptoms, where mass effect and enhancement are common.
The high sensitivity (and specificity) of MR findings can be attributed to the greater use of FLAIR and diffusion MRI (Figs. 14.39 and 14.40) (44). DWI changes have been observed as early as 1 month after the onset of symptoms and may show modifications 6 months prior to T2-weighted images and 4 months prior to FLAIR images. The mechanism of hyperintensity on DWI found in the cortex and basal ganglia is poorly understood, although it correlates with deposition of abnormal prion protein, vacuolation, neuronal loss, and gliosis. DWI can, in some particular examples, precede the onset of characteristic clinical disease. Of interest, primary sensorimotor cortex is almost always spared, even when extensive abnormalities are found in the frontal and parietal cortex. However, more recently, reports using quantitative analysis of apparent diffusion coefficients have shown involvement of the “spared” areas. DWI hyperintensity may disappear in later stages when atrophy ensues, possibly related to gliosis (45), and DWI findings are not necessarily found in all affected areas of a given patient. T1 hyperintensity may be seen in the globus pallidi, may be related to prion protein deposition. MRS has shown diminished levels of NAA neuron marker in advanced disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree