Intracranial Vascular Malformations and Aneurysms
Nicholas A. Telischak
Huy M. Do
Since the last edition of this chapter, magnetic resonance imaging (MRI) has firmly established itself in depiction of lesions of the cerebral vasculature. In addition to now routine standard techniques, many new sequences are pushing the envelope such that physiologic information such as cerebral blood flow is easily acquired noninvasively. It is well established that MRI is the most specific and sensitive noninvasive modality for the detection of intracranial vascular malformations of all types, whether angiographically demonstrable or angiographically occult. Even the detection of subarachnoid hemorrhage (SAH), for which computed tomography (CT) was previously felt to be superior (1), has been shown to be equally or better depicted by MRI (2,3). MR is widely held to be more sensitive to the documentation of the sequelae of vascular lesions and complications of their treatment than CT. As a general rule, MR has replaced CT as the screening modality of choice for intracranial vascular malformations and their complications in all clinical settings, although CT will still prove useful when artifact plagues MRI or when a patient has a contraindication to MRI.
In certain clinical settings and pathologies, it has even become apparent that the complete preoperative assessment of some types of vascular malformations (generally those without arterial or high-flow components), as well as all secondarily related intracerebral pathology, can be made with MR alone. The continued refinement and development of high-field (3 Tesla [T] and beyond) MR scanners, newer pulse sequences, such as diffusion tensor imaging (DTI) and arterial spin labeling (ASL), and improvements to MR angiography (MRA) promise even more sensitive and sophisticated methods for detecting and specifically defining these lesions based on anatomic and pathophysiologic criteria and temporal evolution. Functional MR (fMRI) (see Chapter 33) techniques are no longer nascent and are well suited to the regional mapping of brain functions in the presence of vascular malformations that occupy “eloquent” cortex (4). The role of fMRI in the study of vascular malformations, for both preoperative mapping and more detailed investigations of anomalous development of cortical representations in the presence of congenital space-occupying lesions like arteriovenous malformations (AVMs), has been better elucidated as the techniques have matured.
Although MR has become part of the standard workup of patients suspected (or known) to harbor vascular lesions, catheter angiography continues to be the definitive imaging modality and is still the mainstay of diagnosis in the preoperative and postoperative evaluations of AVMs and aneurysms. Multidetector CT angiography (CTA) has had a significant effect on the imaging of aneurysms, but for vascular malformations, especially AVMs, CTA lacks the temporal resolution accurately to depict these lesions (5). Concurrent with the improvements in MR have been dramatic improvements in CTA, such that both modalities are now complimentary. It should be emphasized that despite all of the improvements in these techniques, intracranial aneurysms in particular still cannot be definitively excluded by any noninvasive modality, including CTA, leaving conventional intra-arterial catheter angiography as the gold standard for aneurysm diagnosis. Three-dimensional (3D) rotational angiography has improved the diagnostic quality and safety of intracranial diagnostic and endovascular treatment procedures for both AVM and aneurysm diagnosis and therapy. The 3D rotational angiography images are obtained by reconstruction of a series of images that are acquired while the C-arm rotates in a continuous movement around the region of interest. Computer-automated reconstruction algorithms then enable the physician to view vascular morphology in any direction, including cut-planes and “flythrough” modes (6,7,8,9,10). The 3D digital subtraction angiography (DSA) and rotational C-arm CT images are now obtainable in nearly all rotational angiography units and are indispensable in the neurovascular setting (11,12).
Vascular Malformations
Vascular malformations of the brain have been classified by McCormick (13) and Russell and Rubinstein (14) into four major pathologic types: AVM, cavernous malformation, capillary telangiectasia, and developmental venous anomaly (DVA). The basis for these classifications is that each entity has its distinct pathologic abnormalities. In addition, each has its unique clinical presentation, treatment, and, in most cases, MR characteristics. Although not typical, clinical imaging studies and necropsy specimens have demonstrated that mixed vascular malformations having pathologic characteristics of two or more of the major types may also occur (15,16,17,18,19).
Aside from these congenital (developmental) lesions, a distinct entity is the dural arteriovenous fistula (DAVF). The DAVF represents an acquired vascular lesion, characterized by arteriovenous (AV) shunting involving vessels within the dural venous sinuses and coverings of the brain. Estimates of the overall incidence of vascular malformations involving the brain range from 0.1% to 4%.
Arteriovenous Malformations
The most common clinically symptomatic cerebrovascular malformation is the AVM. AVMs have an estimated incidence of
about one-seventh that of intracranial aneurysms (20), which corresponds to approximately 0.14% of the population. AVMs represent congenital anomalies of blood vessel development and result from preservation of direct communication between arterial and venous channels without an intervening capillary network (21).
about one-seventh that of intracranial aneurysms (20), which corresponds to approximately 0.14% of the population. AVMs represent congenital anomalies of blood vessel development and result from preservation of direct communication between arterial and venous channels without an intervening capillary network (21).
The focus of all therapy involving any surgical or catheter treatment in the management of a patient harboring an AVM is the tangle of abnormal vessels representing the site of this primitive communication—the nidus—which replaces the normal arterioles and capillaries with a low-resistance, high-flow vascular bed (Fig. 11.1). The nidus permits increased flow through the arterial feeding vessels to the AVM and delivers increased blood volume under relatively high pressure into the cerebral venous system. Therefore, it is of paramount importance in pretherapy imaging to delineate this vascular nidus in those patients for whom intervention is planned.
Clinical Features
Although they are congenital, AVMs most commonly are not clinically apparent until the second through the fourth decades of life, with most having become symptomatic by the time the patient reaches age 40 years (22). In adults, the most common initial symptom is related to acute intracranial hemorrhage, although larger AVMs are more likely to present with seizures rather than acute hemorrhage (23). Seizures and progressive neurologic deficits follow hemorrhage in frequency, and other, less common clinical manifestations may also occur. In those cases in which the AVM becomes apparent in the pediatric age group, hemorrhage is more likely than seizures to be the initial clinical event (24).
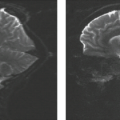
From historical literature, intracranial hemorrhage heralds the existence of the AVM in 30% to 55% of patients and most often occurs during the second or third decade (25,26,27,28). More than 70% of patients who become symptomatic due to acute intracranial hemorrhage do so before age 40 years. Intracranial hemorrhage associated with AVMs is most often intraparenchymal, with the presumed site of bleeding being the nidus or proximal arterialized venous drainage. Intraventricular hemorrhage and SAH may also occur, although AVMs represent the etiology of only some nontraumatic SAHs. On the other hand, the occurrence of nontraumatic isolated intraventricular hemorrhage (i.e., without SAH) in an adult should always suggest the presence of an underlying AVM (Fig. 11.2).
The rate of bleeding from AVMs has received much attention in the clinical literature for decades, and the appropriate management of unruptured AVMs has now become newly controversial. It was generally believed that the incidence of bleeding from cerebral AVMs was much lower than that of intracranial (saccular) aneurysms. Over the past couple of decades, data, however, had suggested a high rate of hemorrhage, in the range of 2% to 4% per year. It had become conventional wisdom that the rate of hemorrhage from an AVM is at least as high as (28) and probably exceeds that of aneurysmal hemorrhage from long-term follow-up studies (26,27). Data suggest that AVMs in deep gray matter have a higher bleed rate than those in other cerebral locations (29). Estimates of annual bleed rates have ranged from 0.9% per year for patients without hemorrhagic AVM presentation, deep location, or deep venous drainage to 34.4% per year in patients having all three risk factors (30).
In 2014, a randomized study (the ARUBA trial) of adults with nonruptured AVMs was published comparing medical
management to interventional plus medical management in 223 patients from 39 sites in 9 countries (31). The ARUBA trial showed that medical management alone is superior to medical management with interventional therapy for the prevention of death or stroke in patients with unruptured brain AVMs followed up for 33 months. The randomized study was halted due to the superior outcome in the medical management alone group. The trial specifically showed a more than threefold increased risk of stroke and death after the initiation of interventional therapy compared with medical management alone in these patients. At the time of this writing, the continued funding of a longer-term study was denied, partly based on the disparity in outcome events between the two treatment arms in the trial. Statisticians calculated a range of 12 to 30 years might be needed for events in the medical arm to reach that of the intervention group, assuming no further events occur in the intervention group. As J.P. Mohr wrote in May 2015, “the current status of the data from ARUBA leaves unsettled both the long-term rate of hemorrhage and hemorrhage severity for those in the medical arm,” but concluded “In adult patients (>18 years) with an unruptured AVM, addition of interventional therapy (ie, neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination) to medical management alone (ie, pharmacologic therapy for neurologic symptoms as needed) is probably harmful” (32).
management to interventional plus medical management in 223 patients from 39 sites in 9 countries (31). The ARUBA trial showed that medical management alone is superior to medical management with interventional therapy for the prevention of death or stroke in patients with unruptured brain AVMs followed up for 33 months. The randomized study was halted due to the superior outcome in the medical management alone group. The trial specifically showed a more than threefold increased risk of stroke and death after the initiation of interventional therapy compared with medical management alone in these patients. At the time of this writing, the continued funding of a longer-term study was denied, partly based on the disparity in outcome events between the two treatment arms in the trial. Statisticians calculated a range of 12 to 30 years might be needed for events in the medical arm to reach that of the intervention group, assuming no further events occur in the intervention group. As J.P. Mohr wrote in May 2015, “the current status of the data from ARUBA leaves unsettled both the long-term rate of hemorrhage and hemorrhage severity for those in the medical arm,” but concluded “In adult patients (>18 years) with an unruptured AVM, addition of interventional therapy (ie, neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination) to medical management alone (ie, pharmacologic therapy for neurologic symptoms as needed) is probably harmful” (32).
 FIGURE 11.2 Isolated intraventricular hemorrhage on sagittal (A) and axial images (B–D) was due to proven arteriovenous malformation. |
From the literature prior to the ARUBA study, each occurrence of hemorrhage from an AVM is associated with a mortality of 10% to 15%, with an overall annual mortality rate in the range of 1%. In addition, permanent neurologic deficit associated with hemorrhage has been estimated to be approximately twice the risk of death, that is, 20% to 30% per episode of hemorrhage, for an annual incidence in the vicinity of 2%. The risk of rebleeding after the initial hemorrhage from cerebral AVM has been estimated to be 6% during the first year (33). After the first year, the rebleeding rate decreases to that of the rate of initial hemorrhage in patients with symptomatic AVMs who had no clinical history of bleeding, estimated to be 2% to 4% per year (20,25). In the analysis of the ARUBA study for patients followed up without interventional therapy, the data demonstrated a low spontaneous rupture rate of 2.2% per year (95% CI, 0.9% to 4.5%). In the past, no significant increase in the risk of AVM hemorrhage was considered to be associated with hypertension or specific situations, such as physical activity, pain, or trauma (20,25,34). However, others have found that hypertension is positively associated with hemorrhage in patients harboring intracranial AVMs (35).
The dominant single predictor of future AVM hemorrhage is initial hemorrhagic presentation; that is, if a patient originally presents with hemorrhage, they are at higher risk of repeat hemorrhage than a patient with an identical-appearing, unruptured AVM. Several anatomic and physiologic factors are associated with increased hemorrhage from an AVM: exclusively deep venous drainage, periventricular/intraventricular or basal ganglia location of the AVM, arterial supply from perforating vessels or from the vertebrobasilar system, intranidal aneurysm(s), and high intranidal pressure, which is reflected by high pressures in the feeding arteries or restriction of venous outflow (36,37,38,39). In a recent large, individual patient data meta-analysis of three large AVM databases, identified risk factors for hemorrhage included hemorrhagic presentation (hazard ratio 3.86), increasing age (hazard ratio 1.34 per decade), female sex (hazard ratio 1.49), and exclusively deep venous drainage (hazard ratio 1.60) (40). The ARUBA study secondary analyses argue against a predictive spontaneous hemorrhage effect from the Spetzler–Martin AVM grade, however. Although investigators have reported that smaller AVMs (less than 2.5 cm) present more frequently with hemorrhage than larger ones, the absolute effects of small size may be overestimated. Smaller malformations may be less likely to cause other symptoms such as seizures, headaches, and steal phenomena than are larger AVMs and therefore are more likely to present with bleeding (41). A high incidence of underlying AVM has been reported by many investigators in patients presenting with intracranial hemorrhage after cocaine abuse (42,43), and so the diagnosis must be aggressively pursued in that specific clinical setting.
Seizures are also a common clinical manifestation of intracranial AVMs, reported as an initial symptom in 20% to 60% of cases in several large series (20,23,33,44). More often associated with AVMs situated in the temporal and frontal regions, seizures affect more than half of AVM patients younger than 30 years.
Acute or progressive neurologic deficits may result from the presence of an intracranial AVM. Although acute neurologic deficits have been reported to accompany 90% of AVM-associated intraparenchymal hemorrhages, neurologic deficits may arise in the absence of bleeding. In fact, the risk of significant morbidity and mortality is high in AVMs, whether or not the lesion has ruptured. Crawford et al. (26) followed 217 patients who had conservative management of their AVMs for a mean period of more than 10 years. They estimated that aside from the risk of hemorrhage, the risk of seizure disorder was 18% and the risk of neurologic dysfunction was 27% during the 20-year follow-up in conservatively treated patients. In the study by Anderson et al. (45), there was a 25% risk of a patient becoming disabled because of intellectual impairment, even without hemorrhage.
Progressive and transient deficits have been ascribed to a number of potential pathophysiologic mechanisms. Among those proposed is “steal” of blood flow from adjacent normal regions of brain into the low-resistance, high-flow vessels feeding the AVM. Dilation of arterial supply to the AVM or enlarged draining veins may result in mass effect with resultant compression and neurologic dysfunction. Hydrocephalus may develop, either as a result of prior hemorrhage or by compression of adjacent cerebrospinal fluid (CSF) pathways. Venous hypertension represents an additional cause of neurologic dysfunction that may affect brain adjacent to or at a distance from the AVM nidus.
Headache is another frequently described clinical manifestation of intracranial AVMs; it affects more than half of patients at some time during their clinical course (46). Although no characteristic headache pattern is consistently observed in AVM patients, a number of authors have reported atypical migraine-like pain with associated visual complaints. The incidence of true migraine, however, appears no higher in patients with AVMs than in the general population. AVMs with arterial supply from dural arteries may cause headache as a result of involvement of the pain-sensitive dura. Other mechanisms of pain include increased intracranial pressure, hemorrhage, hydrocephalus, and mass effect.
Additional clinical associations of AVMs include subjective bruit, which was noted in nearly 30% of patients in one series (25). Objective cranial bruit is an infrequent finding in adult patients with AVMs. Compression of cranial nerves is a rare symptom, most often reflected by atypical facial pain from involvement of cranial nerve V. Hemifacial spasm and glossopharyngeal neuralgia due to involvement of the seventh and ninth nerves have also been described (46).
Pathologic Findings
Although AVMs can be found throughout the central nervous system, intracranial AVMs are located in the supratentorial compartment in approximately 80% to 93% of cases. Supratentorial AVMs usually arise over the convexities and involve the distribution of the middle cerebral artery (MCA), typically visible over the surface of the cerebral hemisphere. However, deep-seated lesions are not uncommon. When AVMs are situated within deep structures, their venous drainage typically
enlarges the deep venous system; in children, this may result in a massive enlargement of the vein of Galen (“vein of Galen aneurysm”), which should not be confused with a vein of Galen malformation, a misnomer that describes an AV fistula occurring in infants and children resulting in an enlarged draining median vein of the prosencephalon. AVMs are most often solitary lesions, but they can be multiple when part of certain syndromes (47), including hereditary hemorrhagic telangiectasia (Rendu–Osler–Weber disease) and mesencephalon-oculor facial angiomatosis (Wyburn–Mason syndrome).
enlarges the deep venous system; in children, this may result in a massive enlargement of the vein of Galen (“vein of Galen aneurysm”), which should not be confused with a vein of Galen malformation, a misnomer that describes an AV fistula occurring in infants and children resulting in an enlarged draining median vein of the prosencephalon. AVMs are most often solitary lesions, but they can be multiple when part of certain syndromes (47), including hereditary hemorrhagic telangiectasia (Rendu–Osler–Weber disease) and mesencephalon-oculor facial angiomatosis (Wyburn–Mason syndrome).
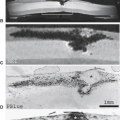
On direct inspection, the gross pathologic appearance of an AVM is a tangled cluster of irregularly dilated vessels with varying wall thicknesses and luminal sizes (Fig. 11.3). Classically, AVMs appear as wedge-shaped clusters of vessels, with the apex of the wedge directed toward the ventricular surface and the base located at the cortical margin. Intervening brain parenchyma is not found within the vascular nidus itself, but feeding and draining vessels are separated by parenchyma when examining the malformation in its entirety (48).
Typically, neither displacement nor mass effect on adjacent structures is present unless hemorrhage has occurred or there has been development of large venous varices involved in the drainage of the lesion. With time, feeding arteries of the AVM gradually enlarge with increased flow, and venous drainage pathways undergo progressive dilation and tortuosity. Approximately 10% of AVMs have associated arterial aneurysms, most of which occur on arteries hemodynamically related to the lesion (49,50). In one recent series, more than 98% of aneurysms originated from arteries hemodynamically or anatomically related to the AVM (50).
On histopathologic examination, arterial channels in AVMs usually show well-defined elastic laminae, a feature absent in the venous channels. Wall thickening of both arterial and venous channels is often present with hyperplasia of smooth muscle cells, fibroblasts, and connective tissue. In many cases, focal areas of wall thinning may be found, representing sites of possible hemorrhage. Regions of thrombosis and recanalization are often present. Intervening and adjacent brain parenchyma frequently exhibit degenerative changes, seen as mild or extensive gliosis and demyelination (Fig. 11.4), often with concomitant parenchymal atrophy (48,51). Evidence of prior hemorrhage is also frequently present, as evidenced by ferritin, hemosiderin, and other iron-storage forms (Fig. 11.5). Calcification may involve not only vessel walls, but also adjacent brain parenchyma due to chronic ischemia or old hemorrhage (Fig. 11.4).
Pretreatment Grading of Arteriovenous Malformations
Grading systems have been developed in an effort to rank individual AVMs into groups predictive of the difficulty associated with a specific treatment and the probable response to that treatment. Ideally, such a system should be sufficiently simple for easy application yet comprehensive enough to permit grading of all AVMs. It should, therefore, encompass all features of the lesion that influence risks of the specific treatment modality and accurately predict the degree of risk associated with the treatment. Although such an ideal grading system does not exist, a number of systems have been proposed.
Surgical grading scales are based on clinical, anatomic, and/or physiologic characteristics of the AVM and the patient. Features studied have included the age and sex of the patient, the presence of neurologic deficits, and the occurrence of prior hemorrhage. The number and location of feeding arteries, site and size of the AVM nidus, and pattern of venous drainage have also been included in various grading systems (52,53,54,55,56).
A relatively simple and widely used AVM grading system (although not widely used in diagnostic neuroradiology reports) is that proposed by Spetzler and Martin (55). This system assigns a numerical grade to the AVM, with higher grades indicating lesions that are more surgically difficult. It requires
evaluation of three features: the size of the nidus, the location of the nidus, and the venous drainage pattern. The nidus size is scored as small (less than 3 cm), medium (3 to 6 cm), or large (greater than 6 cm), with 1, 2, or 3 points given, respectively. Venous drainage is categorized as either superficial (score of 0) if drainage is entirely into the cortical venous system or deep (score of 1) if any or all drainage enters the deep system. The location of the nidus is determined to be within either “eloquent” (score of 1) or “noneloquent” (score of 0) regions of brain, where eloquent areas are those with readily identifiable neurologic function and resultant disabling neurologic deficit when injured (Table 11.1). By this definition, eloquent areas include sensorimotor, visual, or language cortex; internal capsule; thalamus; hypothalamus; brainstem; cerebellar peduncles; and deep cerebellar nuclei (Table 11.2).
evaluation of three features: the size of the nidus, the location of the nidus, and the venous drainage pattern. The nidus size is scored as small (less than 3 cm), medium (3 to 6 cm), or large (greater than 6 cm), with 1, 2, or 3 points given, respectively. Venous drainage is categorized as either superficial (score of 0) if drainage is entirely into the cortical venous system or deep (score of 1) if any or all drainage enters the deep system. The location of the nidus is determined to be within either “eloquent” (score of 1) or “noneloquent” (score of 0) regions of brain, where eloquent areas are those with readily identifiable neurologic function and resultant disabling neurologic deficit when injured (Table 11.1). By this definition, eloquent areas include sensorimotor, visual, or language cortex; internal capsule; thalamus; hypothalamus; brainstem; cerebellar peduncles; and deep cerebellar nuclei (Table 11.2).
TABLE 11.1 Spetzler–Martin Determination of Arteriovenous Malformation (AVM) Grade | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The numerical score from these features is added to give the overall grade of the AVM. For instance, a grade I lesion would be small, located in noneloquent cortex, and have only superficial venous drainage. A grade V AVM would be large, involve eloquent cortex, and have deep drainage. In this system, large or diffuse AVMs encompassing the entirety of critical structures are classified as grade VI because surgical resection of such lesions would be associated with unavoidable disabling neurologic deficit or death.
Therapy
Because AVMs have been thought to hold a grim prognosis if left untreated, the goal of management (in the appropriate clinical setting) has been complete obliteration of the nidus for cure.
Various therapeutic arms are available for patients with intracranial AVMs, including surgery, intravascular embolotherapy, and radiotherapy, often used in some combined approach. Traditional treatment for AVMs has been surgical excision of the nidus. Complete surgical excision can be achieved in approximately 80% of AVMs with mortality and morbidity better than that arising from the natural history of the lesion if left untreated. Combined operative morbidity and mortality rates equal approximately 10% when taking into account all AVMs, regardless of grade (28). With the ARUBA study, however, the treatment of unruptured AVMs is now newly controversial.
Various therapeutic arms are available for patients with intracranial AVMs, including surgery, intravascular embolotherapy, and radiotherapy, often used in some combined approach. Traditional treatment for AVMs has been surgical excision of the nidus. Complete surgical excision can be achieved in approximately 80% of AVMs with mortality and morbidity better than that arising from the natural history of the lesion if left untreated. Combined operative morbidity and mortality rates equal approximately 10% when taking into account all AVMs, regardless of grade (28). With the ARUBA study, however, the treatment of unruptured AVMs is now newly controversial.
TABLE 11.2 “Eloquent” Areas of Braina | ||||||
|---|---|---|---|---|---|---|
|
Most postoperative morbidity in the series by Heros and Korosue (28) occurred in very large AVMs, AVMs that were immediately adjacent to critical anatomy, and AVMs with deep venous drainage (i.e., those that would be classified as Spetzler grade V). If one only considered AVMs of grades I through IV, morbidity and mortality were only 4.5% in the series by Heros and Korosue. To evaluate the proposed Spetzler–Martin grading system, Hamilton and Spetzler (57) prospectively studied 120 patients with brain AVMs who underwent surgery and showed no permanent deficits in grade I, II, and III patients. For grade IV and V patients, permanent neurologic deficits were found in 21.9% and 16.7%, respectively. This AVM grading system accurately correlated with both new temporary (p < 0.0001) and new permanent (p = 0.008) neurologic deficits. Thus, this evaluation confirms the accuracy and utility of this grading system for assisting with management decision-making. Because the rates of immediate rebleeding and mortality and morbidity associated with hemorrhage from AVMs are lower than those associated with aneurysmal hemorrhage, acute or emergent surgical intervention is limited to patients with life-threatening intracranial hemorrhage. Timing of surgery is determined by the characteristics of the lesion and the judgment of the surgeon. Depending on the risk associated with surgical treatment alone, adjunctive or alternative forms of therapy may be used.
Radiosurgery or stereotactic external beam radiation therapy uses focused irradiation directed at the AVM nidus. Radiosurgery is usually pursued in those cases considered unsuitable for resection because of either location of the AVM nidus or overall operative risk. Generally, the size of the nidus must be less than 3.5 cm for the AVM to be considered suitable for treatment by these methods. Radiotherapy techniques cause obliteration of the nidus secondary to radiation damage to vessel endothelium (58), with minimal radiation exposure of the surrounding brain parenchyma. A single dose is used that is larger than the typical fractionated doses used to treat brain tumors. Obliteration rates in the range of 75% to 90% have been reported with permanent neurologic complications (due to radiation necrosis) in the range of 3% to 10% (59,60,61,62). Significant controversy over the role of radiotherapy for AVMs continues, however, as some studies have questioned the efficacy of any form of radiotherapy based on worse reported outcomes from radiotherapy than from microsurgery in some hands (63). Among the advantages of radiotherapy are its relatively noninvasive nature (for some radiosurgical systems stereotactic frames need to be placed on the patient’s skull while others utilize a thermoplastic mask) and its absence of visible effects on the head. Unlike microneurosurgical resection, the effect of radiosurgery takes months to years. Therefore, the risk of hemorrhage is still present until the lesion entirely disappears (64).
Endovascular treatment is usually an adjunctive measure to either surgery or radiation. Complete endovascular obliteration of brain AVMs occurs in only approximately 5% of cases and in general occurs in AVMs that are small and with one or two arterial feeders (65,66,67). Embolization usually precedes surgery or radiosurgery. Surgery most frequently benefits from embolization when deep feeding vessels are eliminated. Reduction in the AVM nidus size decreases venous outflow, which can be helpful, especially when the venous drainage is deep. The goal of preradiosurgical embolization is to reduce the size of the radiosurgical target (AVM nidus) to 3.0 cm or less in all dimensions. The efficiency of AVM obliteration is low when the AVM nidus exceeds 3.0 cm when treated with γ radiation (“gamma knife”) or x-ray photon radiation (“LINAC radiosurgery”). Large AVMs greater than 3.0 cm may benefit from stereotactic heavy–charged-particle Bragg peak radiation (68). Aneurysms associated with AVMs are at risk for rupture before, during, and immediately after the treatment of the AVMs. New aneurysms may arise in patients with high-flow AVMs. The risk of intracranial hemorrhage from either source is higher in female patients. To reduce the complications of intracranial hemorrhage in these patients, these aneurysms should be treated by either surgical or endovascular means before administering definitive therapy for the AVMs (69).
Magnetic Resonance Imaging
Complete imaging evaluation of an AVM requires the acquisition of sufficient information on which to select, plan, and carry out therapy. Features of the lesion to be evaluated include (a) the number, location, and specific identification of arterial supplies to the AVM (including collateral circulation to the AVM and vascular steal from adjacent normal brain); (b) associated vascular lesions (e.g., aneurysms); (c) the presence of hemorrhage (acute or chronic); (d) the location, size, and flow characteristics of the nidus; (e) venous drainage of both the AVM and the normal brain, including the presence of venous thrombosis, outflow restriction, or mass effect; and (f) follow-up of any prior therapy (55,70).
Although cerebral angiography clearly remains the definitive method of fully characterizing the vascular supply and venous drainage of intracranial AVMs, recent advances with MR allow specific diagnosis of these lesions in most cases. It has been recognized that improved anatomic delineation of the AVM nidus, its relationship to vital cerebral structures, and improved definition of the lesion in 3D space with the use of MR (Fig. 11.6) has contributed significantly to optimizing surgical approach (71) and has allowed treatment of some lesions that previously would have been believed to be inoperable (28).
Associated hemorrhage and other parenchymal changes and posttherapy follow-up are best evaluated with the use of MR in conjunction with supplemental MRA. MR information derived from these types of studies is complementary to the angiographic evaluation and clearly contributes significantly to accurate diagnosis and optimal therapeutic decisions (Figs. 11.5 and 11.6).
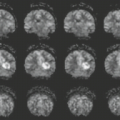
The typical AVM on conventional fast spin-echo or spin-echo (SE) MR is depicted as a cluster of focal round, linear, or serpentine areas of signal void (Figs. 11.4–11.7), representing dilated vascular channels containing relatively rapidly flowing blood (72). Although large high-flow AVMs are usually obvious diagnoses on MRI, subtle enlargement of deep veins occasionally may be the only clue to the diagnosis of these high-flow lesions. Note that certain MR sequences can result in high intensity in areas of flowing blood (Fig. 11.6) because of either flow-related enhancement or even echo rephasing (73) or because of the now routine incorporation of gradient moment nulling into SE imaging. High intensity from these phenomena is generally a reflection of slower flow. Gadolinium enhancement of the enlarged vessels also occurs in those vessels with relatively slow flow, that is, mainly the venous side of the lesion. AVM nidi can partially enhance after gadolinium (Figs. 11.8–11.10), but the rapidly flowing blood within arterial feeding vessels generally does not enhance. Despite the traditional teaching stating that intraparenchymal AVMs demonstrate little or no mass effect on imaging studies unless hemorrhage has occurred, enlargement of draining veins can result in fairly significant mass effect (Fig. 11.10), even in the absence of hemorrhage, in up to one-third of cases (25).
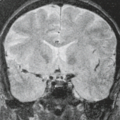
Aside from the features of the intracranial AVM itself, associated findings on MR may provide further insight into the natural history of these lesions and aid in predicting the development of secondary clinical deficits in individual patients. The presence and age of any associated intraparenchymal hemorrhage (Figs. 11.8 and 11.11) and its resultant mass effects are clearly seen on MR. Associated intraparenchymal hemorrhage can be aged on the basis of signal intensity patterns (see Chapter 13). Staining of adjacent brain by iron-storage products can suggest prior subclinical hemorrhage from a clinically asymptomatic AVM. Intraventricular or superficial cortical hemosiderosis from prior (or recurrent) SAHs is a frequent accompaniment to vascular malformations. This entity, not usually seen on CT, is often an incidental MR finding, but patients can develop cranial nerve palsies, most commonly sensorineural hearing loss, or extraventricular obstructive hydrocephalus. Long–repetition
time/echo time (TR/TE) (T2-weighted) SE and gradient-recalled echo (GRE) images demonstrate marked hypointensity along the surface of the brain parenchyma or along the ependymal surface of the ventricle in this entity (Fig. 11.12) (74). Venous occlusive disease, probably an important pathophysiologic feature of many AVMs, should be sought on MR. This can be suggested by massively dilated vessels (nearly always representing veins) of either the deep or superficial venous system. Enlargement of the medullary veins, even in the contralateral hemisphere from the site of the AVM, is an important clue to venous occlusive disease (particularly in dural AV fistulas; see Dural Arteriovenous Fistulas). Signal intensity alterations representing gliosis
and/or secondary demyelination in the vicinity of the AVM are easily demonstrated and imply chronic vascular ischemia, perhaps because of steal from adjacent brain.
time/echo time (TR/TE) (T2-weighted) SE and gradient-recalled echo (GRE) images demonstrate marked hypointensity along the surface of the brain parenchyma or along the ependymal surface of the ventricle in this entity (Fig. 11.12) (74). Venous occlusive disease, probably an important pathophysiologic feature of many AVMs, should be sought on MR. This can be suggested by massively dilated vessels (nearly always representing veins) of either the deep or superficial venous system. Enlargement of the medullary veins, even in the contralateral hemisphere from the site of the AVM, is an important clue to venous occlusive disease (particularly in dural AV fistulas; see Dural Arteriovenous Fistulas). Signal intensity alterations representing gliosis
and/or secondary demyelination in the vicinity of the AVM are easily demonstrated and imply chronic vascular ischemia, perhaps because of steal from adjacent brain.
To determine the precise role of MR in the diagnostic workup of intracerebral AVMs, several investigators have studied patients with these lesions and compared MR findings with those of other imaging modalities (71,75,76). MR has been shown to be competitive with both CT and catheter angiography for demonstrating the neuroanatomic location of the nidus and the relationship of its supplying and draining vessels to deep ganglionic structures, the ventricular system (Figs. 11.6 and 11.13), and the corpus callosum (Fig. 11.14 and 11.15).
This information is critical to treatment planning, whether surgical, endovascular, or radiosurgical (72).
This information is critical to treatment planning, whether surgical, endovascular, or radiosurgical (72).
MR and CT allow for accurate representations of lesion volumes, and are often used as an adjunctive study to both MR and angiography for treatment planning purposes, although the gold standard that we use for radiosurgical planning is that of fused 3D rotational angiograms (Figs. 11.16 and 11.17). The size of the AVM nidus is important for many reasons, including an overall increase in operative grade (and risk) with increasing nidus size and the potential for normal perfusion pressure breakthrough after a large nidus is resected (77). The potential for hemorrhage is also related to AVM size: smaller AVMs tend to present more often with hemorrhage than larger AVMs, perhaps because by definition they are less likely to cause symptoms related to mass effect (78). MR is also superior to CT for demonstrating the degree of nidus obliteration after intra-arterial embolization. In such cases, MR often allows clear depiction of the thrombosed portion of the lesion and accurate differentiation of patent from thrombosed vessels, a distinction that even conventional arteriography may not make with certainty because of the many physiologic changes in flow after endovascular therapy (71). MR is more sensitive than either CT or angiography at delineating hemorrhagic complications of AVMs, especially those that are subacute or chronic (Figs. 11.5 and 11.8).
Conventional catheter angiography remains superior to MR in depicting the specific arterial supply and venous drainage of the AVM. Even thin-section, high-resolution MR can only implicate abnormal veins or arteries in an AVM on the basis of enlargement, a feature that may not be present in all involved vessels. Similarly, 3D time-of-flight (TOF) MRA is a nonselective technique that cannot define with certainty the specific feeding arteries and draining veins, although approaches to selective time-resolved MRA or MR DSA techniques have been developed (Fig. 11.16) (see Chapter 28). These time-resolved techniques include both noncontrast ASL sequences (79), and bolus tracking sequences with gadolinium. Even newer contrast agents such as ultrasmall superparamagnetic iron oxide particles (USPIO) (e.g., Ferraheme) may further improve what MR can show (80). Currently, the spatial and temporal resolution of these sequences still cannot match a catheter directed DSA. Moreover, one must detect and characterize aneurysms that are associated with the AVM because certain types are reported to have an extremely high risk of rupture (81). Obliteration of symptomatic intranidal or perinidal aneurysms is now considered a part of the surgical or endovascular management of AVMs (50,69), and so these lesions must be defined on imaging studies (Figs. 11.13, 11.17–11.19). If MRA is performed in these patients, then the region of interest is not limited to the AVM itself and must include the circle of Willis and feeding arteries into the nidus.
Differential Diagnosis
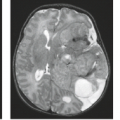
Because AVMs are frequently associated with intracranial hemorrhage, the question of the diagnosis is often raised when the patient presents after an episode of intracranial hemorrhage. In the presence of any intracerebral hematoma, the radiologist must search for evidence of large vessels to suggest AVM, which can be detected with either CT or MR (Fig. 11.20). It is important to realize that the failure to identify large vessels on MR in the presence of an acute hematoma does not entirely exclude an AVM as the cause of the hemorrhage (Fig. 11.21). Small AVMs
occasionally can be angiographically occult because of a variety of factors, including compression of the lesion by adjacent hematoma, vasospasm, extremely slow flow, and thrombosis. These lesions are often referred to as “cryptic” AVMs.
occasionally can be angiographically occult because of a variety of factors, including compression of the lesion by adjacent hematoma, vasospasm, extremely slow flow, and thrombosis. These lesions are often referred to as “cryptic” AVMs.
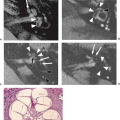
 FIGURE 11.21 Hyperacute hematoma with cryptic arteriovenous malformation (AVM). A: Sagittal T1-weighted magnetic resonance (MR) (600/11). B: Axial T2-weighted MR (3,000/80). C: Lateral view, arterial phase, angiogram. D: Anteroposterior view, arterial phase, angiogram. Parietal mass (A,B,1) represents hyperacute hematoma (see Chapter 9), with no definite evidence of AVM on MR. Arteriogram shows posterior parietal AVM with small nidus (C,D, closed arrows) and draining vein (D, open arrows). |
A major part of the role of the radiologist once the identification of an intracerebral hemorrhage has been made on MR is the search for any associated enlarged vessels because the diagnosis of an underlying AVM is of paramount importance in these cases. A point of confusion may arise when a cavernous hemangioma is identified but vascular channels are noted in contiguity with the lesion (Fig. 11.22). The keys to the diagnosis of the “mixed malformation,” composed of cavernous hemangioma plus venous angioma (discussed later in this chapter), are the recognition of two features of the associated vessels: the characteristic “spoke-wheel” morphology of the enlarged vessels and the very slow flow through these vessels (shown by either SE intensities or with gadolinium enhancement) (Figs. 11.23 and 11.24).
Occasionally, a hypervascular neoplasm with markedly enlarged vessels manifests as an acute hematoma. The only potential source of confusion with AVM might be a hemangioblastoma, which can certainly be associated with markedly enlarged vascular channels and intraparenchymal hematoma. The typical macrocystic component and the identification of the enhancing mural nodule of hemangioblastoma separate this entity from AVM in most cases. Moreover, hemangioblastomas do not generally bleed.
Although the diagnosis of AVM is usually unambiguous on MR, one other lesion can superficially masquerade as an AVM because it too is depicted as abnormally dilated vascular channels. Moyamoya disease is characterized by the occurrence of progressive symmetric occlusion involving the bifurcations of the internal carotid arteries (ICAs) and the proximal anterior and middle cerebral arteries. The occlusive process stimulates the development of an extensive network of enlarged basal, transcortical, and transdural collateral vessels. The angiographic appearance of the innumerable tiny collateral vessels, termed “puff of smoke” or “moyamoya” in Japanese, gives the condition its name.
Moyamoya disease has a bimodal age presentation, with the first peak occurring in the first decade of life, associated with cerebral infarction as progressive carotid occlusion develops. Adult patients most often present in the fourth decade with intracranial hemorrhage arising from the rupture of the delicate network of collateral vessels. Hemorrhage from moyamoya is intraparenchymal in 60% of cases, with intraventricular hemorrhage accounting for nearly all the rest (82,83). Occasionally, isolated SAH is the initial manifestation of the disease.
 FIGURE 11.22 Hemorrhage involving dorsal medulla cannot be dismissed as cavernous malformation due to subtle vessels in adjacent subarachnoid space, later proven as arteriovenous malformation. |
Characteristically, flow voids representing large collaterals and the markedly enlarged striate vessels are seen on MR amid the deep ganglionic structures (84,85). Absence of the expected flow void within the cavernous and supraclinoid portions of the ICAs is a consequence of narrowing and ultimately occlusion of these vessels. Changes resulting from ischemia or rupture of the fragile, enlarged network of collateral vessels, including regions of infarction or hemorrhage, may also be seen (Fig. 11.25). This diagnosis is clearly defined by MR when evidence of the occlusive disease of the distal ICAs is apparent in conjunction with the flow voids representing the enlarged striate vessels. Associated enhancing leptomeningeal collaterals after gadolinium and hyperintensity within these surface collaterals and small arterial branches on FLAIR images should suggest this diagnosis.
Most often, the underlying occlusive process is idiopathic and is more commonly seen in Asian patients. An identical picture may result from progressive distal internal carotid occlusion arising from any other etiology that is accompanied by collateral development, termed moyamoya syndrome. Such causes include neurofibromatosis and sickle cell disease and as a delayed effect of radiation therapy to the suprasellar region. An increased incidence of moyamoya changes may also be present in patients with Down syndrome. Another entity associated with marked enhancement of surface vessels that should not be confusing is Sturge–Weber syndrome (Figs. 11.26 and 11.27). On close inspection, the MR changes associated with moyamoya are usually quite characteristic and should not be confused with the findings of an AVM or any other entity.
Posttherapy MR
In addition to the initial pretreatment evaluation of the patient harboring an intracranial AVM, MR has gained increasing importance in the assessment of these patients after treatment has been completed. Several investigators have studied the effects of radiosurgery on intraparenchymal AVMs based on imaging (86,87,88). Expected pathologic changes after therapy include deposition of collagen within the subendothelial space of the nidus, resulting in gradual narrowing and thrombosis of the lesion. A significant reduction in nidus size after therapy is usually clearly recognized on MR, even without MRA. This is depicted as a decrease in previously recognizable flow void with increased signal intensity on T2-weighted images and persistent contrast enhancement in the area of the nidus. Significant evidence of reductions in AVM flow generally does not occur until at least 12 months after the initial treatment (Fig. 11.28) (89). It should be noted that CT is inaccurate in evaluating residual AVM nidus size after radiosurgery because persistent contrast enhancement is demonstrated even after complete obliteration.
Complications of this treatment may also be monitored by MR. Changes in the surrounding parenchyma have been noted as early as 3 months after treatment and include transient vasogenic edema and radiation necrosis. Most often, asymptomatic vasogenic edema is a frequent finding in these patients and is characterized by hyperintensity on T2-weighted images in white matter surrounding the nidus. Symptomatic radiation necrosis occurs in an estimated 3% to 4% of patients and is seen as high intensity with mass effect and irregular enhancement (Fig. 11.29). Hemorrhage can be concomitant with radiation necrosis.
In an in vitro study, liquid cyanoacrylate mixtures used for superselective endovascular embolization of AVMs demonstrated signal characteristics similar to fat secondary to the iophendylate component that is added to make the mixture more radiopaque. After the addition of blood to simulate in vivo postembolization and polymerization conditions, the signal characteristics of clotted blood predominated (90). MRI of patients after staged transarterial flow-directed embolization with N-butyl cyanoacrylate demonstrated the region of the “glue” cast as mixed regions of alternating high and low signals on conventional images (Figs. 11.30–11.32).
Specialized MR Techniques in Arteriovenous Malformations
MR Angiography
Since the initial publication by Wedeen et al. (91) showing intravascular flow as high signal intensity on in vivo MR images, intense scientific and clinical interest has focused on imaging the cerebral vasculature and its pathology with MR. The various methods of imaging vascular flow and displaying it in multiple projections have been termed “MR angiography” because of the similar appearance of these images to conventional catheter angiography using iodinated contrast agents and x-rays. MRA for the brain and spine consists of three principal techniques of data acquisition: TOF, phase contrast (PC), and contrast-enhanced TOF (92,93,94,95,96,97,98).
The major impetus for the development of MRA as a potential replacement for catheter angiography in the diagnosis of neurologic diseases is the morbidity and mortality of cerebral catheter angiography. The historical literature reports that aside from local complications due at the puncture site itself, there is approximately a 4% incidence of neurologic event, a 1% fixed neurologic deficit incidence, and a small but definable (less than 0.1%) incidence of death from these procedures (99). It should be recognized, however, that in competent and well-trained hands, cerebral angiography is well documented to be a safe procedure and has become much safer with the improvement in equipment, contrast agents, and techniques (100,101). In fact, in a meta-analysis of the risk of angiography in patients with SAH, cerebral aneurysm, and AVMs, Cloft et al. (102) found that the risk of permanent neurologic complication is only 0.07%, much lower than that found in patients presenting with transient ischemic attacks or ischemic stroke (0.7%; p = 0.004). The second motivation for developing MRA for the workup of neurologic disease, particularly in the United States in the current healthcare climate, is the hospital cost of catheter angiography, a factor that varies widely and is too complex a subject for this review.
More specific characterization of regions of signal void on (fast) SE images as regions of blood flow rather than from dense calcification or hemorrhage can be obtained by using gradient-refocused limited flip-angle techniques (94,103). These techniques form the basis of TOF and PC MRA (see Chapter 16). On these sequences, regions of flowing blood are most often demonstrated as high signal intensity. These techniques can be relatively rapid methods of clarifying ambiguous regions of signal intensity on SE images as flowing blood (such as subependymal vessels or vessels near cortical margins). The presence of major venous sinus occlusion accompanying the AVM can also
be clarified with MRA techniques. It is clear that at least some familiarity of the physics of flow is essential to the appropriate design and implementation of these techniques (104) (see Chapter 32). While not generally thought of as an MRA sequence, blood pool imaging with 3D postcontrast acquisitions (e.g., BRAVO) is excellent at delineating the venous side of the cerebral circulation and can be performed either following gadolinium contrast or USPIO which has a strong T1 shortening effect and yields exquisite images of the blood pool (Fig. 11.8).
be clarified with MRA techniques. It is clear that at least some familiarity of the physics of flow is essential to the appropriate design and implementation of these techniques (104) (see Chapter 32). While not generally thought of as an MRA sequence, blood pool imaging with 3D postcontrast acquisitions (e.g., BRAVO) is excellent at delineating the venous side of the cerebral circulation and can be performed either following gadolinium contrast or USPIO which has a strong T1 shortening effect and yields exquisite images of the blood pool (Fig. 11.8).
The fundamental role for such flow imaging techniques as MRA in the diagnostic workup of the patient with an AVM is controversial at this point in the evolution of these techniques (105). Interesting early results have been reported on the utility of 3D volume-rendered surface anatomy displays of 3D TOF MRA for staging of AVMs (106,107,108). This display technique has been used together with catheter cerebral angiography for the treatment planning and targeting of AVMs for radiosurgery.
Although no one doubts that MRA can demonstrate the vascular nature of a high-flow AVM, the importance of simply showing the tangle of vessels on an image is arguable because the mere identification of the lesion is usually accomplished even with conventional MR. In fact, the AVM can sometimes be even more clearly separated from adjacent hematoma by MR rather than by MRA (Figs. 11.11 and 11.31). MRA in those AVMs that are fed by enlarged dural vessels is a valuable tool because dural feeding arteries are often not seen well on conventional SE MR because of their intrinsic signal void superimposed against the background of signal void of skull base and calvarium. The search for dural vessels involved in AVMs is a clear indication for supplemental MRA rather than MR alone (see Dural Arteriovenous Fistulas).
One could make a strong argument that the assessment of AVM nidus size might be an appropriate indication for MRA of these lesions, whether pretreatment or posttreatment, for several reasons. First, in terms of prognosis, many neurosurgeons classify AVMs at least in part based on nidus size (55,78). Second, it is common practice to evaluate these patients after radiotherapy and/or embolization to determine the residual nidus, particularly if one desires to avoid exposing these patients to invasive catheter angiograms multiple times. Third, most institutions that treat some AVMs with a form of radiotherapy triage these lesions based on the size of the nidus, among other factors, where lesions less than 3.5 cm are more difficult to treat with such therapy (59,60,61,62). MRA does represent a potentially effective way to demonstrate a quantifiable nidus size in 3D space (Fig. 11.32). Reports of the utility of time-resolved projectional MRA and dynamic MR DSA for depiction of intracranial AVM and dural AVM have been encouraging (109,110,111). Recently, a comparative study demonstrated that 3D TOF MRA at 3 T is superior to the same technique at 1.5 T in terms of image quality, detection rates of feeding arteries, and draining veins (112). More important, however, the same study found that both MRA techniques are equal in nidal size evaluation and both are inferior to DSA in all assessment criteria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree