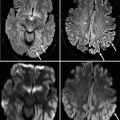
Fig. 20.1
The bony skull base, without and with colored areas, denoting the frontal (blue), central (green), and posterolateral (red) regions
Diseases that affect the skull can be intrinsic to the area or due to extension from either side of the divide. Intracranial lesions, in particular those affecting the pituitary gland and the meninges, can extend downward to involve the skull base (Fig. 20.2a, b). Several deep spaces of the neck, the masticator, carotid, parapharyngeal, retropharyngeal, and perivertebral spaces, are intimately related to the skull base. As such, diseases in these spaces can spread directly to the skull base. There are several apertures in the skull base that allow cranial nerves and brain vasculatures to traverse the deep spaces of the neck. These can act as conduits for transmission of diseases from the deep spaces of the neck into the intracranial compartment and vice versa.
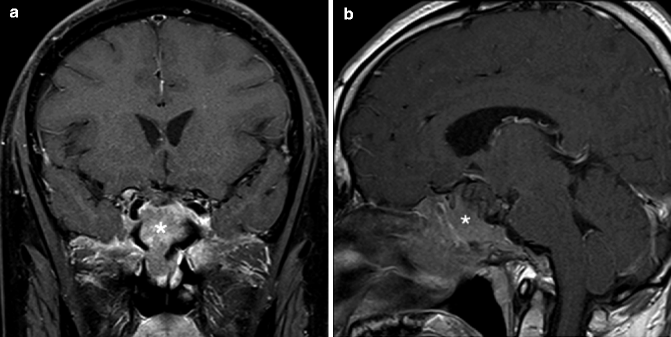

Fig. 20.2
(a) Coronal and (b) sagittal post-contrast fat-saturated T1-weighted MR images of a patient with pituitary adenoma (*) showing invasion of the skull base and extension into the sphenoid sinus
Clinical assessment of skull base lesions is limited, as only the superficial aspects of these lesions are accessible to visual examination. Radiological examination is required for delineation of the full extent of these lesions. Radiography has a very limited role in the investigation of skull base in the current day context and has largely given way to computed tomography (CT) and magnetic resonance (MR) imaging. The evolution of CT and MR imaging has enabled precise determination of the extent of skull base lesions and, in cases of tumors, has allowed preoperative planning to maximize the chance of complete resection while minimizing morbidity. CT is superior to MR imaging in the determination of cortical bone involvement and demonstration of calcification, although MR imaging is conversely superior to CT in the assessment of meningeal, bone marrow, and cranial nerve involvement, and is less affected by dental amalgam, which can cause beam-hardening artifacts that degrade CT images. MR imaging is also slightly better than CT in the delineation of soft tissue extent.
The decision on which of the two should be the first choice for skull base lesions depends on several factors. If the lesion primarily involves bony structures, e.g., lesions affecting the temporal bone, CT should be the first choice. Many would prefer MR imaging to CT for lesions which involve soft tissues, bone marrow, and the intracranial compartment. However, the availability of MR imaging and the cost of the examination can influence the decision to use CT instead. On some occasions, both CT and MR imaging is needed, with MR imaging offering better delineation of the extent of the lesion and CT providing information on important anatomical bony landmarks for preoperative planning. The diseases that affect the skull base in the geriatric population can be categorized into broad groups: infection/inflammatory, neoplastic, traumatic, otodystrophies, and fibro-osseous lesions.
20.2 Infection/Inflammation
20.2.1 Sinusitis
The imaging findings of acute sphenoid or frontal sinusitis include the presence of an air–fluid level (Fig. 20.3a) of the involved sinuses. Acute frontal sinusitis, in particular, may lead to intracranial complications such as subdural empyema, epidural or intracerebral abscess, and meningitis (Fig 20.3b) (Giannoni et al. 1997), sometimes as early as within 48 h (Remmier and Boles 1980) of onset. The presence of an air–fluid level in the frontal sinus would therefore require prompt and vigorous treatment with antibiotics.
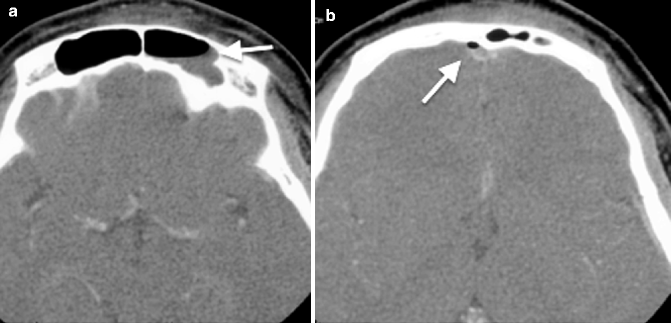

Fig. 20.3
Axial CT images taken at two different levels show (a) left frontal sinusitis with an air–fluid level (arrow), complicated by (b) subdural empyema with an air pocket (arrow)
Acute sphenoid sinusitis, due to the close relationship of the sphenoid sinus to adjacent neurovascular structures, can result in cavernous sinus thrombosis and extracranial complications such as superior orbital fissure syndrome and orbital apex syndrome (Fig. 20.4a, b). The clinical presentations of both are those of diplopia, paralysis of extraocular motion, exophthalmos, and ptosis, with visual loss in the latter. Such complications are, however, rare in present day practice with widespread availability and usage of antibiotics. Cavernous sinus thrombosis can be identified on contrast-enhanced CT and MR imaging by filling defects within an enlarged and laterally bulging cavernous sinus (Fig. 20.4b). In chronic sinusitis, there may be mucosal thickening, which may be associated with sclerosis of the sinus wall on CT in chronic cases.
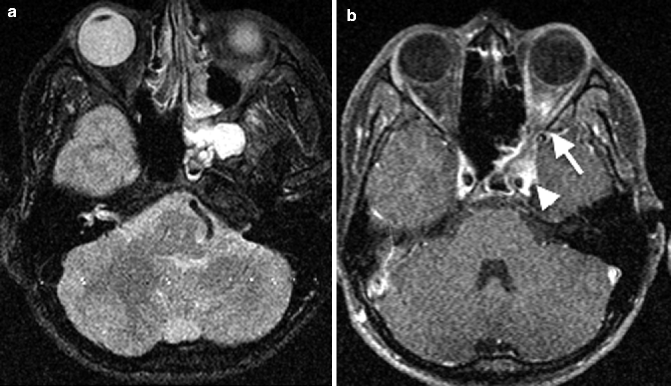

Fig. 20.4
Axial (a) fat-saturated T2-weighted and (b) post-contrast fat-saturated T1-weighted MR images show left sphenoid sinusitis with orbital apex involvement (arrow) and left cavernous sinus enlargement (arrowhead) with a filling defect due to cavernous sinus thrombosis
Inflammatory obstruction of the ostium could result in a mucocele, which is a dilated mucous membrane-lined sac containing mucoid secretions. This presents as a non-enhancing, low-density mass that completely fills the affected sinus on contrast-enhanced CT, with expansion and remodeling of the bony wall (Fig. 20.5). With MR imaging, the mucocele may be variably hyperintense on T1-weighted images, depending on the protein content, as well as hyperintense on T2-weighted images, due to the water in the secretions (Dillon et al. 1990). However, if the mucocele has become inspissated, the signal would become very hypointense on both T1-weighted and T2-weighted images, to the extent that it might be mistaken for air (Dillon et al. 1990).
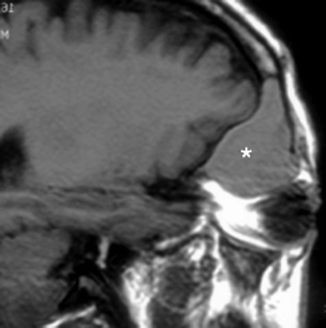

Fig. 20.5
Sagittal T1-weighted MR image shows a frontal mucocele (*) with hyperintense signal
On CT, a mucocele with inspissated secretions would be seen as a high-density mass within the affected sinus. Of the mucoceles involving the paranasal sinuses, the frontal sinus is the most commonly affected (Toriumi et al. 1988; Cagigal et al. 2006), with the sphenoid sinus affected infrequently. The clinical presentation of mucoceles results from the effect of their expansion into the surrounding structures. Frontal sinus mucoceles tend to expand inferiorly into the orbits. As such, proptosis and diplopia are common presentations of frontal sinus mucoceles (Cagigal et al. 2006). Sphenoid sinus mucoceles can expand in one direction or concentrically in all directions (Toriumi et al. 1988). Structures at risk from the expansion of a sphenoid mucocele include the cavernous sinuses (the carotid arteries and cranial nerves III, IV, V, VI), the pituitary gland, optic nerves and optic chiasm, superior orbital fissure, and the posterior orbit. Hence, sphenoid sinus mucoceles tend to present with multiple cranial neuropathies and visual loss without orbital signs.
Fungal infections of the sinuses can be invasive or noninvasive. Both forms can occur in immunocompetent or immunocompromised patients, although the invasive form is more common in the latter. Aspergillus species is the most common organism responsible for the noninvasive form. It usually presents as an intractable sinusitis that fails to respond to repeated courses of antibiotics and the presence of foul-smelling, purulent, and pasty material within the affected sinuses (deShazo et al. 1997).
Imaging findings of noninvasive fungal sinusitis can be nonspecific and similar to that of chronic sinusitis: nodular mucoperiosteal thickening and sinus wall sclerosis on CT. Foci of hyperdensity, attributed to calcium or metal components in the mycetoma, have been reported in CT of fungal sinusitis (Fig. 20.6a), although the presence of thick pus or thrombus can on occasion exhibit similar findings. MR imaging findings of fungal sinusitis are more specific, with hypointensity on T1-weighted images and marked hypointensity on the T2-weighted images (Fig. 20.6b) (Zinreich et al. 1988).
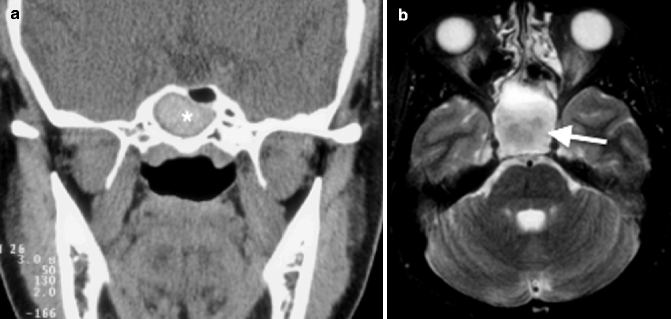

Fig. 20.6
Fungal sinusitis. (a) Coronal CT image shows a rim of low-density mucus surrounding the dense concretion centrally (*) from the sinus wall. (b) Axial T2-weighted MR image shows hypointense contents (arrow)
The invasive form of fungal sinusitis is more often secondary to mucor species than Aspergillus species. Histopathological studies have shown fungal invasion of blood vessels, including the carotid arteries and the cavernous sinuses, leading to vasculitis with thrombosis, hemorrhage, and tissue infarction (deShazo et al. 1997). Imaging findings of invasive fungal sinusitis include multiple areas of focal destruction of the sinus wall (better appreciated on CT than MR imaging), cavernous sinus thrombosis, carotid artery thrombosis, and dural enhancement (Fig. 20.7).
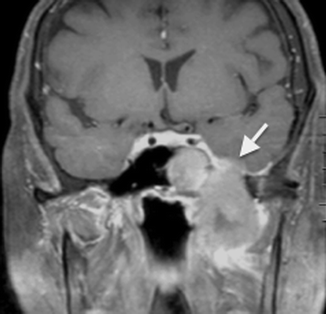

Fig 20.7
Coronal post-contrast fat-saturated T1-weighted MR image shows invasive fungal sinusitis with dural thickening and enhancement in the left middle cranial fossa (arrow)
20.2.2 Necrotizing Otitis Externa
Necrotizing otitis externa, or malignant otitis externa, as it has been formerly known, is a disease which classically affects elderly diabetic patients, although it can also affect other immunocompromised patients. If left untreated, the disease can be fatal. This is predominantly attributable to Pseudomonas aeruginosa, although other possible pathogens include Staphylococcus epidermidis and fungi. The clinical presentation is usually that of otorrhea and severe otalgia, with persistent granulation tissue in the external auditory canal, at the bony–cartilaginous junction.
The disease usually begins as an otitis externa which later involves the bony walls of the external auditory canal, spreading through the fissures of Santorini, to involve the soft tissue of the deep neck spaces beneath the skull base and the temporomandibular joint (Fig. 20.9). Posterior extension into the mastoid, medial extension into the middle ear, and the petrous apex are other routes of spread. Skull base osteomyelitis is an advanced stage of the disease. Facial nerves and other cranial nerve palsies indicate a poor prognosis. Intracranial involvement, which may result in meningitis, abscess formation, and sigmoid sinus thrombosis, is the most frequent cause of death in these patients.
Radionuclide studies such as technetium-99m (Tc-99m) and gallium-67 citrate are sensitive in the initial detection of the disease but lack anatomical definition.
In most imaging centers, the initial assessment is performed with CT or MR imaging. CT findings of NEO include soft tissue in the external auditory canal, opacification of the middle ear cavity and mastoid air cells, erosion of the inferior wall of the external auditory canal, and, in more extensive disease, destruction of the clivus or petrous apex with soft tissue thickening and/or abscesses in the prevertebral, masticator, carotid, and parapharyngeal spaces. Imaging findings on MR imaging are similar, although it is less sensitive than CT in the detection of bony erosion. However, marrow edema and intracranial involvement, such as dural enhancement and sigmoid sinus thrombosis, are better delineated by MR imaging (Grandis et al. 1995).
The spread of the disease into the deep spaces of the neck beneath the skull base can result in a bulge in the nasopharynx. This can result in the occasional radiological misinterpretation of nasopharyngeal carcinoma (NPC) with skull base invasion, especially in regions where the latter is prevalent. There are several helpful radiological features for distinguishing between the two. The mucosal surface of the involved nasopharynx is smooth in NEO, because the disease extends to the submucosal layer and does not involve the mucosal layer (Fig. 20.8a). In NPC, the mucosal layer is usually irregular, apart for an uncommon subtype that arises from the submucosal layer. Involvement of the temporomandibular joint is common in NEO (Kwon et al. 2006) but is rare in NPC (Fig. 20.9).
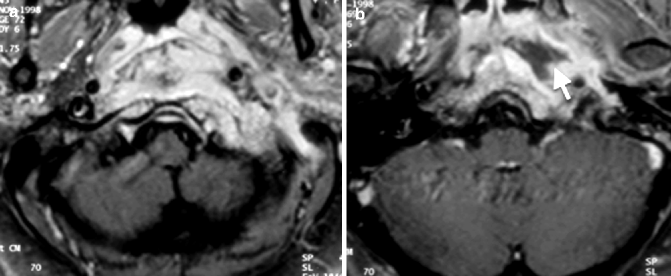
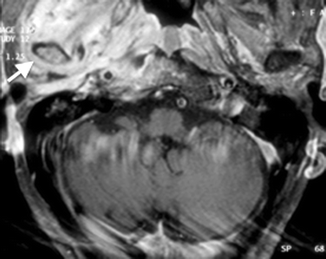

Fig. 20.8
Necrotizing otitis externa. Axial post-contrast fat-saturated T1-weighted MR images show (a) a mass in the left posterior nasopharynx involving the skull base with a smooth mucosal lining. (b) An abscess is also evident as a rim-enhancing hypointense collection (arrow)

Fig. 20.9
Axial post-contrast fat-saturated T1-weighted MR image shows right-sided necrotizing otitis externa with anterior extension and involvement of the right temporomandibular joint (arrow)
The presence of an abscess, which indicates an infective process (Fig. 20.8b), is therefore likely to be indicative of NEO. In NPC, the usual presentation is a solid mass. On MR imaging, inflammatory soft tissue shows abnormal signal changes although the underlying parenchymal architecture is usually preserved in NEO. In NPC, the architecture of involved soft tissues is often distorted due to tumor infiltration. Lymphadenopathy is a common presentation in NPC. The usual sites of involvement are the retropharyngeal space, carotid space, and posterior cervical space. Lymphadenopathy is not usually seen in NEO and, when present, may involve the periauricular lymph nodes. Clinical profiles of the patient and laboratory findings indicative of infection are also useful in differentiating between the two.
Treatment of NEO requires long courses of antibiotics. Ascertaining eradication of the disease before cessation of treatment is thus very important. CT and MR imaging are excellent in the demonstration of resolution of the soft tissue component of NEO, but changes of the bone often lag behind clinical resolution. This is because reversal of bony changes requires a long interval following clinical resolution of the disease. As such, bone erosions can still be demonstrated on CT months after clinical cure. Also, changes in the bone marrow may persist for months after successful therapy. Therefore, CT and MR imaging cannot be accurately relied on for the determination of treatment termination. Tc-99m is also not useful for this purpose, as increased bone activity due to remineralization may persist for months or even years after clinical cure. As gallium-67 citrate uptake quickly returns to normal once an infection has been cleared, some authors have advocated that this is the best imaging modality to monitor treatment progress and to determine when to cease treatment (Okpala et al. 2005).
20.2.3 Chronic Otomastoiditis
Unlike acute otomastoiditis, which is a pathogenic infection affecting children more commonly than adults, chronic otomastoiditis is caused by eustachian tube dysfunction. The resultant decreased intratympanic pressure may give rise to several manifestations that are diagnosable radiologically. These include tympanic membrane retraction and perforation, middle ear effusion, granulation tissue, cholesteatoma, and postinflammatory ossicular fixation (PIOF). CT is the initial imaging modality of choice for these manifestations. Tympanic membrane retraction and perforation are easily seen on otoscopy – these will not be described here. Middle ear effusions are diagnosed on CT by the presence of an air–fluid level, preferably in two orthogonal planes (Fig. 20.10a, b) (Swartz 1983). Differentiation of effusions from other middle ear debris, such as granulation tissue, however, is not possible if the middle ear cavity is completely opacified.

Fig. 20.10
(a) Axial and (b) coronal CT images show air–fluid levels (arrow) in the right middle ear
Granulation tissue is the most common cause of opacification in the middle ear cavity. It is usually present as either typical granulation tissue or a cholesterol granuloma. The latter contains cholesterol crystals and is the result of repeated hemorrhage and subsequent foreign body response by multinucleated giant cells (Plester and Steinbach 1982). Both types of granulation tissue present on CT as a nondependent opacity in the middle ear cavity without mass effect and in uncomplicated causes, without bony erosion (Fig. 20.11). A cholesterol granuloma should be suspected if there is a bluish lesion seen behind the tympanic membrane on otoscopy. Both types of granulation tissue can be differentiated radiologically on MR imaging. Typical granulation tissue is isointense, and cholesterol granuloma is hyperintense on non-contrast T1-weighted sequences (Fig. 20.12) (Miglets and Booth 1981).
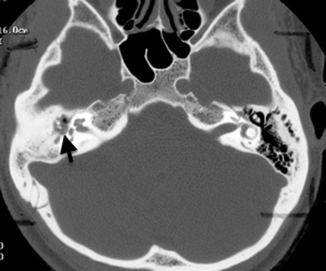
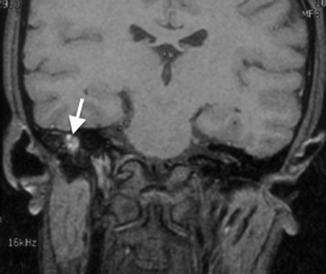

Fig. 20.11
Axial CT image shows granulation tissue in the right middle ear, seen as an opacity without mass effect or bone erosion (arrow)

Fig. 20.12
Coronal T1-weighted MR image shows a cholesterol granuloma in the right middle ear, depicted as a hyperintense focus (arrow)
A cholesteatoma consists of a sac which is lined by keratinizing stratified squamous epithelium filled with exfoliated debris. This may be thought of as “skin in the wrong place.” The outer layer of the tympanic membrane is contiguous with the skin of the external auditory canal. The skin on the tympanic membrane grows faster, desquamates, and continuously migrates outward, together with the cerumen that is produced in the cartilaginous portion in the lateral third of the external auditory canal (Moran 1980). The tympanic membrane has two portions: the pars flaccida and the pars tensa. The pars flaccida or Shrapnell membrane is the small triangular flaccid portion that makes up the peripheral aspect of the tympanic membrane. The pars tensa is the thick and taut portion that contributes to the central portion of the tympanic membrane.
There are several theories on the development of cholesteatomas, the most widely accepted being that cholesteatomas develop from retraction pockets. The negative pressure in the middle ear cavity due to eustachian tube dysfunction may result in a retraction pocket in the tympanic membrane, most commonly in the superior pars flaccida portion and less commonly in the pars tensa portion. As the retraction pocket deepens, possibly due to repeated inflammation, the normal physiological migration of the desquamated skin is interrupted, with accumulation of keratinized debris. Subsequent moisture aggregation causes an expansion of the debris resulting in the development of a cholesteatoma (Ruedi 1983).
A pars flaccida cholesteatoma is located between the malleus and the superior attachment of the tympanic membrane. It expands superiorly into the epitympanum, often resulting in a medial displacement of the malleus and incus (Fig. 20.13). The retraction pocket in which a pars tensa cholesteatoma arises from is usually in the posterosuperior aspect of the middle ear cavity. A pars tensa cholesteatoma tends to extend medially to the malleus and incus toward the mastoid antrum, displacing these ossicles laterally (Fig. 20.14).
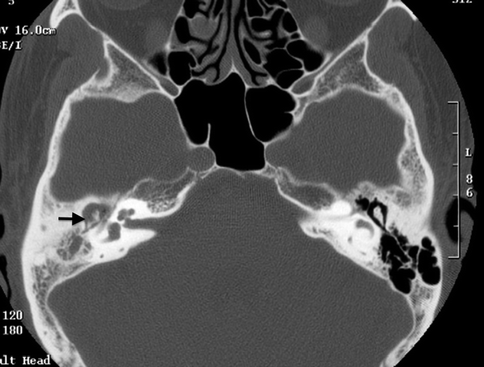
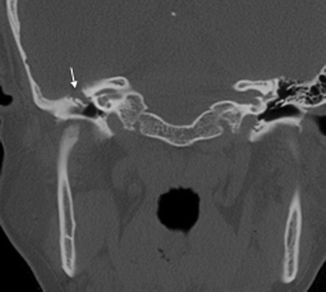

Fig. 20.13
Axial CT image shows a pars flaccida cholesteatoma with medial displacement of the ossicles as well as erosion of the body of the incus (arrow)

Fig. 20.14
Coronal CT image shows a pars tensa cholesteatoma with lateral displacement of ossicles as well as erosion of the tegmen tympani (arrow)
The diagnosis of cholesteatoma can usually be made clinically when squames or a pearly white mass behind the tympanic membrane is seen on otoscopy (Buckingham 1982). Radiologically, the diagnosis of cholesteatoma can be made when there is a nondependent mass, in a typical location associated with bone erosion or ossicular destruction. Such groupings, however, are only seen in 50 % of cholesteatomas (Jackler et al. 1984). When there is difficulty distinguishing between granulation tissue and cholesteatoma on the CT scan, MR imaging can play a valuable role. Non-enhancement in the post-contrast scan and demonstration of restricted diffusion of cholesteatoma allow it to be differentiated from granulation tissue on MR imaging (De Foer et al. 2010).
Complications of cholesteatomas are secondary to bony erosions. These include ossicular erosions, labyrinthine fistula formation, and facial nerve dysfunction. Ossicular erosions lead to conductive hearing loss. The long process of the incus is the most commonly affected part of the ossicles involved by both varieties of cholesteatomas. Erosion of the stapes is more commonly seen in pars tensa cholesteatomas, whereas erosion of the malleus head and incus body are more common with the pars flaccida variety. Labyrinthine fistulas most frequently involve the lateral semicircular canal (Fig. 20.15).
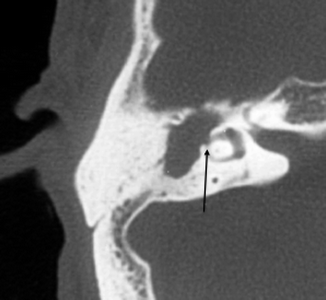

Fig. 20.15
Axial CT image shows a cholesteatoma causing labyrinthine fistula (arrow) of the lateral semicircular canal
Much less commonly seen is a cochlear fistula due to erosion of the promontory (Chao et al. 1996). A patient with a labyrinthine fistula usually presents with intermittent vertigo associated with long-standing ear discharge. A telltale clinical sign is when an acute vertiginous episode is elicited during otoscopic manipulation of the cholesteatoma. Facial nerve dysfunction occurs in approximately 1 % of cholesteatoma patients (Telischi et al. 1995). This is usually due to erosion of the tympanic portion of the facial nerve. Erosion of the sinus plate (Fig. 20.16) may lead to sigmoid sinus thrombosis.
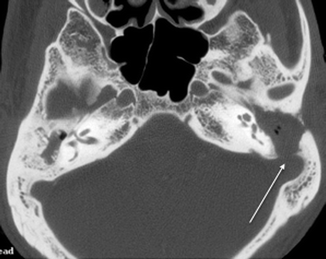

Fig. 20.16
Axial CT image shows a cholesteatoma causing erosion of the sigmoid plate (arrow)
PIOF may cause conductive hearing loss in the absence of ossicular erosion. It has been classically divided into three pathological types: fibrous, hyalinization of collagen, and fibro-osseous sclerosis (Swartz et al. 1985). The term “tympanosclerosis” was previously used for all three entities. The fibrous type of PIOF has a nonspecific appearance on CT, with generalized or a focal nonerosive soft tissue opacity within the middle ear cavity that cannot be differentiated from granulation tissue. If focal, it is most commonly seen at the region of the oval window. The tympanic membrane is usually retracted. Clinical suspicion of this entity should be raised if there is a wide audiometric air–bone gap (usually greater than 30 dB), more than that expected in the absence of ossicular disruption.
The hyalinization of collagen type is the result of fibroblastic invasion of submucosal layers, with subsequent thickening and fusion into a homogenous mass with deposition of calcium and phosphate crystals (Swartz et al. 1985). It most commonly involves the tympanic membrane (Fig. 20.17a, b), although in isolation, this usually does not give rise to significant conductive hearing loss. Conductive hearing loss results when the process encases the ossicles or involves their suspensory ligaments and tendons. Fibro-osseous sclerosis or new bone formation is the least common of the three entities, most commonly affecting the epitympanum, with encasement of the ossicles (Fig. 20.18a, b).
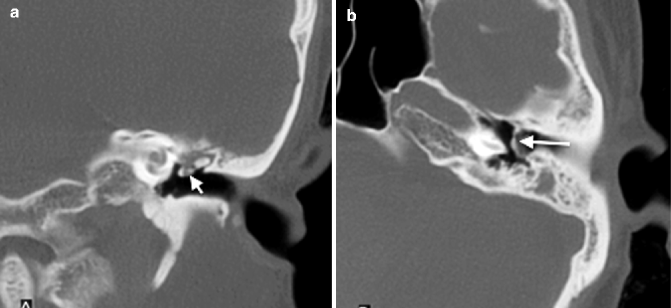
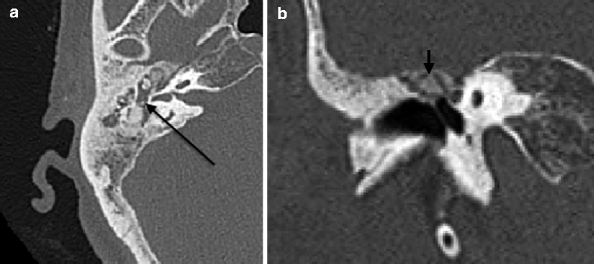

Fig. 20.17
(a) Coronal and (b) axial CT images show a retracted tympanic membrane with calcification (arrows)

Fig. 20.18
(a) Axial and (b) coronal CT images show fibro-osseous PIOF, with opacification of the middle ear (a, arrow) and epitympanic bone formation (b, arrow)
20.2.4 External Auditory Canal Cholesteatoma
Cholesteatomas in the external auditory canal are less common than in the middle ear cavity. The typical patient is between 40 and 75 years old and may present with persistent ear discharge and chronic dull otalgia. The disease is usually unilateral. Otoscopy may show focal erosion in the osseous portion of the external auditory canal, with an accumulation of squamous debris, granulation tissue associated with periostitis, and necrotic bone. In contrast to middle ear cholesteatomas where eustachian tube dysfunction plays a significant causative role, the etiology of external auditory canal cholesteatomas is largely unknown, although it is believed to be the sequelae of periostitis. On CT, it presents as a soft tissue mass in the osseous portion of the external auditory canal with adjacent bony erosion. Tiny bony densities secondary to necrotic bone and periostitis may be seen within the soft tissue (Fig. 20.19).
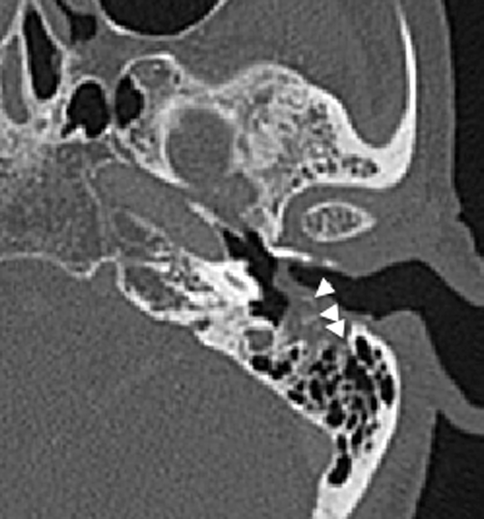

Fig. 20.19
Axial CT shows an external auditory canal cholesteatoma with bony erosions and bony spicules (arrowheads) representing periostitis and necrosis
20.3 Trauma
Skull base fracture is uncommon, occurring in only about 4 % of cases of severe head injury (Graham and Gennaveli 2000). Most such injuries are the result of automobile accidents, violence, sports, or falls from high places. However, as accidental falls from slipping or tripping are the most common cause of head injury in the elderly, the occurrence of skull base fracture in this group of patients is rare. CT of the brain is usually the first imaging to be employed, and skull base fracture should be suspected if there is pneumocephalus or opacification of the middle ear cavity/mastoid air cells or if high-density fluid is seen in the sphenoid, ethmoid, or frontal sinuses. In these instances, fine-section multiplanar reformatted images of the skull base using volumetric data or a dedicated scan of the skull base will delineate the fractures.
Fractures of the skull base more commonly involve the temporal bones, orbital roofs, and the posterior basiocciput, rather than the central basicranium. The fractures can cause tears in the meninges, leading to cerebrospinal fluid (CSF) leakage. Leakage into the middle ear cavity can occur and, in the presence of a perforated ear drum, would result in otorrhea. If the tympanic membrane is intact, CSF leakage into the nasopharynx via the eustachian tube may occur, with the patient complaining of a salty taste.
In fractures of the anterior skull base, CSF leakage into the nose can result in CSF rhinorrhea.
The numerous neurovascular structures that traverse the central skull base can be compromised by the fractures, in particular, those that involve the sphenoid body and the basiocciput. A displaced bony fragment can also impinge onto nerves, leading to neurological dysfunction. In particular, the optic canal must be examined carefully, as a displaced fragment from fractures involving the anterior clinoid process or the lateral wall of the sphenoid sinus bordering the canal can impinge on the optic nerve (Fig. 20.20), leading to permanent visual loss if the pressure on the nerve is not alleviated quickly. The muscles of mastication, the extraocular muscles, and the eustachian tubes are attached to the sphenoid bone, and fractures can result in separation of these muscles, leading to functional deficits.
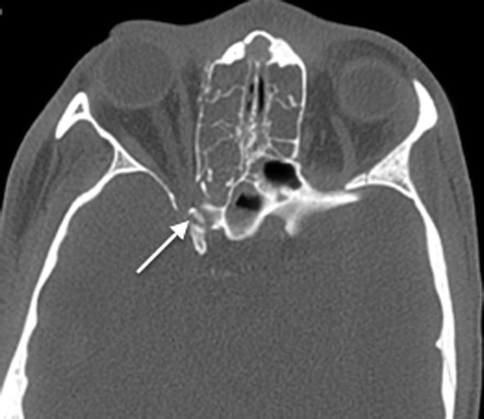

Fig. 20.20
Axial CT image shows a displaced fracture fragment projecting into the optic canal (arrow)
Fractures of the temporal bone follow the path of least resistance offered by the natural fissures and foramina. These fractures are divided into two main groups, longitudinal and transverse fractures, according to their orientation to the long axis of the petrous pyramid (Cannon and Jahrsdoerfer 1983). Oblique and mixed varieties have also been described (Ghorayeb et al. 1987). However, the traditionally accepted grouping of longitudinal and transverse fractures will be used here. Longitudinal fractures, which are more common than transverse fractures, are most often the result of blunt trauma to the temporal or parietal region. These fractures are parallel to the long axis of the petrous pyramid.
Longitudinal fractures are usually extra-labyrinthine and most commonly extend anteriorly in relation to the labyrinth, through the tegmen tympani, anterior to the first genu of the facial nerve, and terminating near the petrous apex. The fractures may extend to the carotid canal and reach the sphenoid sinus. Fractures into the carotid canal can damage the internal carotid artery, leading to dissection, thrombosis, or pseudoaneurysm. Fracture of the lateral wall of the sphenoid sinus may injure the adjacent internal carotid artery, leading to carotid cavernous fistula. Less commonly, longitudinal fractures extend posteriorly in relation to the labyrinth, into the middle ear cavity, through the jugular foramen, and into the posterior cranial fossa. Transverse fractures are perpendicular to the petrous pyramid and are usually the result of blunt trauma to the occiput. The fractures either extend laterally in relation to the arcuate eminence (the convexity subtended by the superior semicircular canal) into the labyrinth or medially into the fundus of the internal auditory canal.
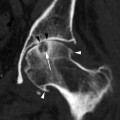
Complications associated with temporal bone fractures include vertigo, hearing loss, facial nerve palsy, and CSF leakage. Vertigo results if there is transection of the vestibule or vestibular nerves, often secondary to transverse fractures. Hearing loss may be sensorineural or conductive, with sensorineural hearing loss more common in transverse fractures, most likely due to fracture involvement of the labyrinth or internal auditory canal (with cochlear nerve transection). Extension of fractures into the labyrinth may result in a perilymph fistula, which is a communication between the labyrinth and the middle ear cavity. Air may also be introduced into the labyrinth, creating a pneumolabyrinth which is readily seen on CT (Fig. 20.21).
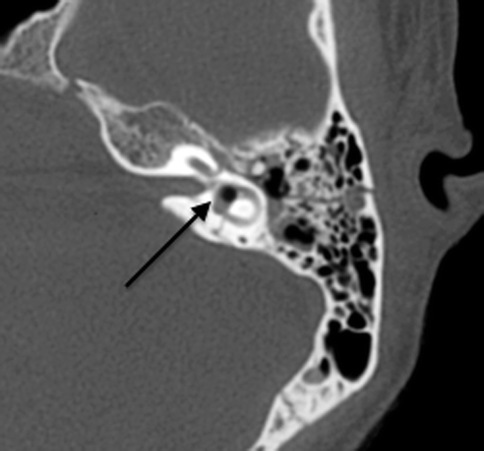

Fig. 20.21
Axial CT shows a pneumolabyrinth (arrow) due to a labyrinthine fistula secondary to fracture
Conductive hearing loss is a common sequela of temporal bone fractures. Hemotympanum and tympanic membrane rupture result in temporary conductive hearing loss, resolving with the resorption of the middle ear debris or with the healing or surgical repair of the tympanic membrane. Ossicular disruption, however, will result in persistent conductive hearing loss. Subluxation of the incudostapedial joint is the most common form of ossicular disruption (Fig. 20.22), followed by malleoincudal subluxation (Fig. 20.23) (Hough 1970), sometimes associated with ossicular fractures. Ossicular disruption is more common with longitudinal fractures.
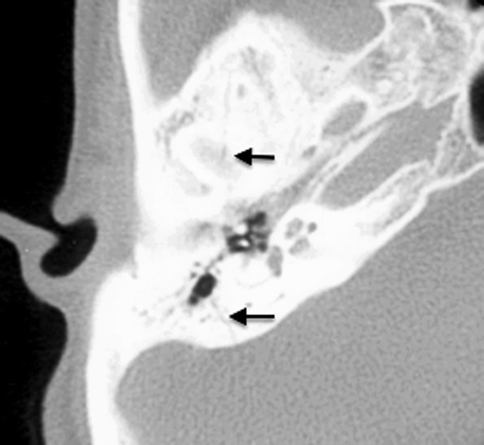
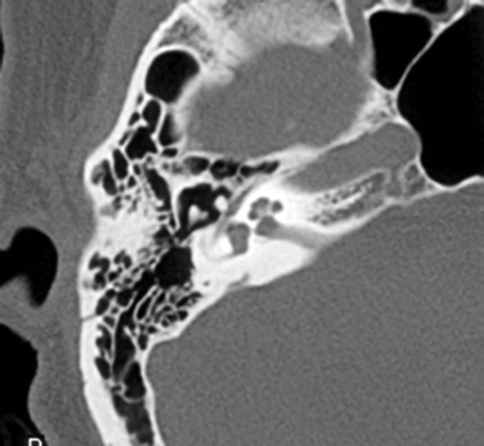

Fig. 20.22
Incudostapedial disruption. Axial CT image shows temporal bone fracture (arrows). This patient presented with a 6-month history of persistent hearing loss post head injury

Fig. 20.23
Axial CT image shows temporal bone fracture with incudomalleal dislocation
Facial nerve palsy could be due to intraneural edema, hematoma, or impingement of the facial nerve by fracture fragments (Fig 20.24) or even complete nerve transection. Intraneural edema or hematoma usually results in a facial palsy of delayed onset, whereas complete transection results in immediate facial nerve paralysis. Both the longitudinal and transverse fractures are associated with facial nerve palsy, with the latter being more common (Cannon and Jahrsdoerfer 1983). CSF leakage is more commonly associated with longitudinal fractures and is due to the involvement of the tegmen tympani or the roof of the middle ear (Fig. 20.25). This may present as otorrhea or rhinorrhea, as previously described.
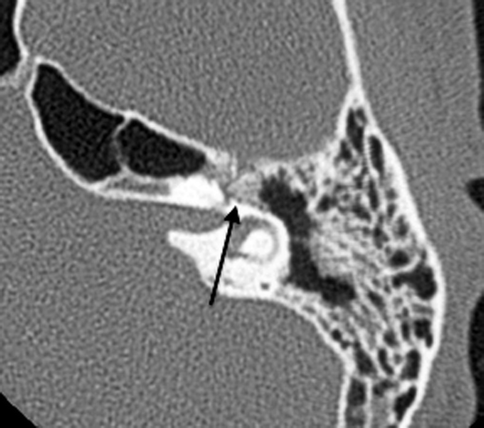
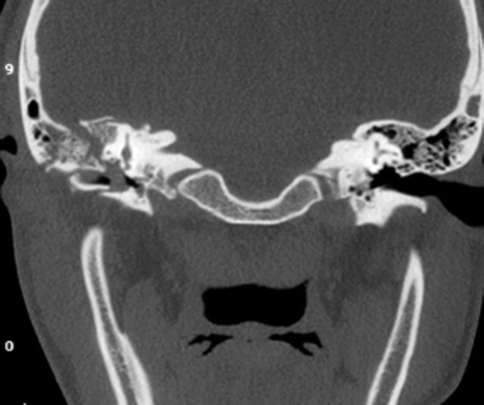

Fig. 20.24
Axial CT shows temporal bone fracture with a displaced bony fragment impinging on the geniculate ganglion (arrow)

Fig. 20.25
Coronal CT image shows right temporal bone fracture involving the tegmen and scutum, with opacification of the right middle ear
20.4 Tumors
Similarly, tumors can arise in or around the skull base, but they generally involve the bony components. In the elderly, the types of tumors are fairly specific, given the demographics. The location and compartment demarcated by the bony skull base can generally help determine the origin of the tumor.
20.4.1 Anterior Skull Base
Sinonasal tract tumors are extremely rare tumors, making up less than 1 % of all malignant tumors and up to 3 % of all head and neck cancers (Goldenberg et al. 2005




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree