Group
T category
N category
M category
Occult carcinoma
TX
N0
M0
Stage 0
Tis
N0
M0
Stage IA
T1a
N0
M0
T1b
N0
M0
Stage IB
T2a
N0
M0
Stage IIA
T2b
N0
M0
T1a
N1
M0
T1b
N1
M0
T2a
N1
M0
Stage IIB
T2b
N1
M0
T3
N0
M0
Stage IIIA
T1a
N2
M0
T1b
N2
M0
T2a
N2
M0
T2b
N2
M0
T3
N1
M0
T3
N2
M0
T4
N0
M0
T4
N1
M0
Stage IIIB
T1a
N3
M0
T1b
N3
M0
T2a
N3
M0
T2b
N3
M0
T3
N3
M0
T4
N2
M0
T4
N3
M0
Stage IV
Any T
Any N
M1a
Any T
Any N
M1b
Table 13.2
The AJCC definitions of TNM for lung
Primary tumor (T) | |
TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
T1 | Tumor 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus)a |
T1a | Tumor 2 cm or less in greatest dimension |
T1b | Tumor more than 2 cm but 3 cm or less in greatest dimension |
T2 | Tumor more than 3 cm but 7 cm or less or tumor with any of the following features (T2 tumors with these features are classified T2a if 5 cm or less) |
Involves main bronchus, 2 cm or more distal to the carina | |
Invades visceral pleura (PL1 or PL2) | |
Associated with atelectasis or obstructive pneumonitis that extends to the hilar region but does not involve the entire lung | |
T2a | Tumor more than 3 cm but 5 cm or less in greatest dimension |
T2b | Tumor more than 5 cm but 7 cm or less in greatest dimension |
T3 | Tumor more than 7 cm or one that directly invades any of the following: parietal pleural (PL3) chest wall (including superior sulcus tumors), diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium; or tumor in the main bronchus less than 2 cm distal to the carinaa but without involvement of the carina; or associated atelectasis or obstructive pneumonitis of the entire lung or separate tumor nodule(s) in the same lobe |
T4 | Tumor of any size that invades any of the following: mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina, separate tumor nodule(s) in a different ipsilateral lobe |
Regional lymph nodes (N) | |
NX | Regional lymph nodes cannot be assessed |
N0 | No regional lymph node metastases |
N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension |
N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) |
N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural (or pericardial) effusionb |
M1b | Distant metastasis |
Table 13.3
Changes in the 7th edition of the TNM classification for lung cancer
Component of the classification | Changes |
|---|---|
T | Subclassification of T1 according to tumor size in: T1a ≤2 cm T1b > 2 cm but ≤3 cm |
Subclassification of T2 according to tumor size in: T2a > 3 cm but ≤5 cm (or tumor with any other T2 descriptors, but ≤5 cm) T2b > 5 cm but ≤7 cm | |
Reclassification of T2 tumor >7 cm as T3 | |
Reclassification of T4 tumors by additional nodule/s in the same lobe of the primary tumors as T3 | |
Reclassification of M1 tumors by additional nodule/s in another ipsilateral lobe as T4 | |
Reclassification of T4 tumors by malignant pleural effusion as M1a | |
N | No change |
M | Subclassification of M1 in: M1a: separated tumor nodules/s in the contralateral lung; tumor with pleural nodules or malignant pleural (or pericardial) effusion M1b: distant metastasis |
The therapeutic strategies for NSLC vary according to histology and staging. The National Comprehensive Cancer Network (NCNN, at www.nccn.com/files/cancer-guidelines/nscl/files/assets/basic-html/page1.html, last accessed July 16, 2012), guidelines recommend surgery, radiation therapy and chemotherapy (either alone or in various combinations, depending on disease status) as the modalities commonly used to treat these patients. Stage I or II confers the best chance for cure, surgery remaining the most important first-line form of treatment for NSCLC up to stage IIIA.
Whereas, surgery is rarely indicated for patients with SCLC, as the standard approach includes cycles of chemotherapy and radiotherapy.
The surgical approach to patients with NSCLC varies according to size and location of the primary tumor. The various types of surgery include: a) wedge resection (removal of a peripherally located tumor tissue and of a small part of the surrounding lung parenchyma), b) lobectomy (removal of a single lung lobe, performed in case of small tumors peripherally located), or c) pneumomectomy (removal of an entire lung, generally performed for larger or more central tumors). Patients with stages I and II NSCLC are candidates for radical curative surgery, although adjuvant chemotherapy and radiotherapy can also be considered in these cases.
Surgery may be useful in patients with stage IIIA NSCLC, although without radical curative intents. Adjuvant chemotherapy is mandatory in these patients, while radiotherapy can be added, during chemotherapy or immediately after. In some of these cases neoadjuvant chemotherapy is employed in order to reduce tumor size in patients with tumors not directly amenable to surgery.
Stages IIIB and IV are generally treated with combined radio- and chemotherapy. Multiagent chemotherapy results in improved survival than single-agent chemotherapy. The cornerstone drug for chemotherapy is platinum and its derivatives (cisplatin and carboplatin), generally combined with other anticancer agents.
Palliative treatments (surgery and/or radiotherapy, combined sometimes with single-agent chemotherapy) can be considered with the intent to relieve symptoms and improve quality of life, when no real goal of cure or cancer remission can reasonably be contemplated due to the advanced stage of the disease.
Conventional Imaging Methods
Imaging plays an essential role in the detection, staging and response assessment. CT is the modality of choice for lung cancer imaging, whereas magnetic resonance imaging (MRI) is used for resolving specific questions, i.e., chest wall or mediastinal invasion, adrenal mass characterization or differentiation between tumor recurrence and radiation fibrosis [3].
Multi-detector CT devices provide a high-quality images with short acquisition times [4]. Thin slices help avoid volume averaging and improve the display of anatomic structures. MDCT data can be reconstructed as axial, sagittal, coronal, and oblique images, as well as multi-planar reformations (MPR), maximum and minimum intensity projections (MIP or MinIP), virtual bronchoscopy, and volume rendering. Low-dose CT can detect stage 1 lung tumors 4–6 time more frequent than conventional radiology [5]. However, some lung cancers, presenting as pulmonary nodules, can be missed on CT [6] due to error in perception or misinterpretation. Subcentimeter nodules and low-density nodules also known as ground glass opacity (GGO) can be missed [7, 8].
Detection of a new solitary pulmonary nodule (SPN) in a patient with a history of lung cancer raises the possibility of recurrent disease. Features such as lesion size, location, contour, edge, and density (including the presence or absence of calcifications or fat) should be evaluated [9, 10]. Unfortunately, none of these features alone establish benignity or malignancy. Specific combinations of features are more likely to be associated with either malignant or benign disease. For example, spiculated borders, ill-defined contours, presence of bubble-like lucency, eccentric calcification, semisolid lesion, or GGO are suggestive of malignancy, whereas a completely solid lesion with smooth and well-defined contours, total, central or popcorn calcification, or inclusions of fat are indicative for benignity [11]. Because of the lack of specificity for diagnosis of malignancy, approximately three quarters of SPNs, especially small lesions, less than 10 mm in a diameter, are classified as indeterminate after performing CT and patients are enrolled in a follow-up program or undergo fine needle aspiration (FNA).
Evaluation of the growth rate is based on calculating the doubling time on two consecutive examinations from measurements of the nodule diameters. It can aid in the characterization, especially of indeterminate nodules. Inter- and intra-observer reproducibility of 2D diameter measurements is low [12]. Automated computation of the size of an SPN on 3D reconstruction has been shown to improve the ability to assess its growth [13], with the exception of nodules that are abutting the chest wall or vessels, when errors can occur [14].
Once the diagnosis of lung cancer is established, a next important work-up step is the assessment of the anatomic extent of the tumor. Staging separates patients who are candidates for surgical resection from those with inoperable disease who should be treated with chemotherapy and/or radiation. Staging is performed according to the recommendations of the International Staging System and previously described evidence-based guidelines are used for clinical purposes [7].
Staging (TNM)
TNM staging has a very important role in managing patients with lung cancer. Treatment options as well as prognosis vary for the different stages of disease. Tumor (T) staging is better accessed by either CT or MRI as these techniques offer better resolution and anatomical details. T status depends on tumor size, location, and relationship to nearby structures. Five-year survival rates have been reported to differ in relationship to the tumor size—77% in T1a (≤2 cm), 71% in T1b (2–3 cm), 58% in T2a (3–5 cm), 49% in T2b (5–7 cm), and 35% in T3 tumors (>7 cm) [13]. Data are now available showing the importance of [18F]FDG imaging in TNM staging [15–18]. [18F]FDG imaging also has a significant role in assessing involvement of the pleura. Pleural fluid cytology can be falsely negative in 30–40% of patients [19]. On the other hand, [18F]FDG imaging has a sensitivity of 89–95%, specificity of 67–94%, and accuracy of 91–92% for the detection of malignant pleural involvement [20, 21].
Loco-regional lymph node spread represents an important factor in lung cancer management, especially in the absence of distant metastases. Patients with either negative or positive N1 (bronchopulmonary or hilar) nodes are considered for lung resection without further testing. Mediastinal lymph node positive [ipsilateral (N2) or contralateral (N3)] patients are usually referred to confirmation biopsy either by mediastinoscopy, endoscopic ultrasound (EUS), or endobronchial ultrasound (EBUS). If positive on further tests, management is changed from surgery alone to a combined approach of surgery with chemo/radiotherapy or combined chemo/radiotherapy without surgery. [18F]FDG imaging has an established high accuracy for nodal staging. NICE [22] suggested that [18F]FDG-negative or a chain of [18F]FDG-positive nodes would not necessarily need confirmation by other diagnostic testing.
In the UK the management of N2 disease varies. Most patients are regarded as not suitable for radical surgery alone and neoadjuvant chemotherapy can be considered in a trial setting. Depending on the nodal station, some patients are better served with radical radiotherapy. In other countries combined modality management is recommended for N2 disease. Proven N3 disease patients are usually not considered for radical treatment procedures. As indicated in the 7th edition TNM classification, patients with single N1 disease have a 5-year survival of 48% as compared to 35% in patients with multiple N1, 34% for single N2, and 20% in patients with multiple N2 nodes [23].
In addition to T and N staging, [18F]FDG PET-CT imaging is useful for M staging where M0 = no metastasis and M1 = distant metastases. The presence of distant metastases usually changes patient management drastically with stage 4 patients typically treated symptomatically and for palliation. Patients with good performance status (e.g., Zubrod/ECOG 0–2) are offered palliative chemotherapy, radiotherapy, and/or active supportive care. The 5-year survival rates in patients with involvement of ipsilateral nodes, pleural effusion, contralateral nodes, and distant metastases are 16%, 6%, 3% and 1%, respectively [23].
These data have lead to changes in lung cancer stage grouping in 2009 (Tables 13.4 and 13.5). The major changes include stage T2 large size was upgraded from IB to IIA, and small T2 N1 was downgraded from IIB to IIA. According to this new TNM staging, the 5-year survival rates for NSCLC are 73% for stage IA, 58% IB, 46% IIA, 36% IIB, 24% IIIA, 9% IIIB, and 13% for stage IV [24].
Table 13.4
Stage grouping by TNM subsets (1997)
T1 | T2 | T3 | T4 | |
|---|---|---|---|---|
N0 | IA | IB | IIB | IIIB |
N1 | IIA | IIB | IIIA | IIIB |
N2 | IIIA | IIIA | IIIA | IIIB |
N3 | IIIB | IIIB | IIIB | IIIB |
M1 | IV | IV | IV | IV |
Table 13.5
New stage grouping by TNM subsets (2009)
T1a | T1b | T2a | T2b | T3 | T4 nodule same lobe | T4 other | T4 nodule ipsilateral lobe | M pl. effusion contralateral nodule | |
|---|---|---|---|---|---|---|---|---|---|
N0 | IA | IA | IB | IIA | IIB | IIB | IIIA | IIIA | IV |
N1 | IIA | IIA | IIA | IIB | IIIA | IIIA | IIIA | IIIA | IV |
N2 | IIIA | IIIA | IIIA | IIIA | IIIA | IIIA | IIIB | IIIB | IV |
N3 | IIIB | IIIB | IIIB | IIIB | IIIB | IIIB | IIIB | IIIB | IV |
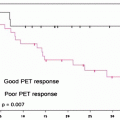
The same TNM classification is also suggested for SCLC. The 5-year survival rates for small cell lung cancer (SCLC) are 38% for stage IA, 21% IB, 38% IIA, 18% IIB, 13% IIIA, 9% IIIB, and 1% for stage IV [25] (Fig. 13.1).
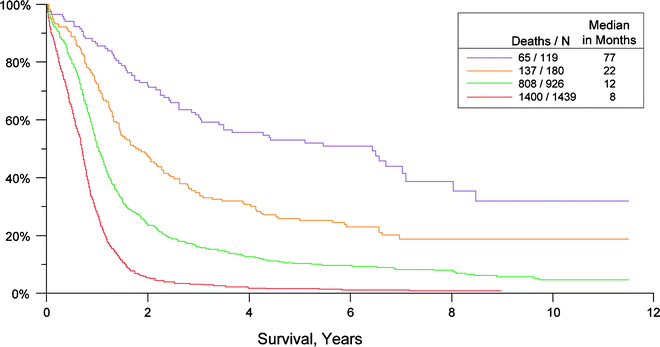

Fig. 13.1
Survival in all SCLC by TNM stage (according to “best” stage based on a combination of clinical and pathologic staging in the IASLC lung database) (Used with the permission of the American Joint Committee on Cancer (AJCC), Chicago, IL. The original source for this material is the AJCC Cancer Staging Manual, Seventh Edition (2010) published by Springer Science and Business Media LLC. www.springer.com)
T Stage
Selection of the appropriate therapeutic approach requires measurement of tumor size and local extent (T stage) of the lesion. CT is an excellent tool for this purpose. The size of the lesion is easily measured and its edges can be identified in most cases. However, the presence of atelectasis or consolidation distal to the tumor can complicate the identification of tumor margins. In the case of an intra-luminal lesion, CT can be useful in identifying its proximal part, put another marker, such as the extent of hypermetabolism is required to define the distal extent of the lesion. When the suspected lesion is surrounded by lung parenchyma thin-section CT is crucial to determine whether the tumor invades the pleura [26]. In the presence of pleural invasion the surgical approach cannot be modified. CT or MRI can be used to detect chest wall invasion but are not helpful in distinguishing chest wall invasion from fibrous adhesions. Reliable diagnosis of chest wall invasion can be made in the presence of rib destruction or of an obvious chest wall mass [27, 28]. The problem remains unresolved in the presence of contiguity of the tumor with the parietal pleura or of pleural thickening. The accuracy of CT for diagnosis of chest wall invasion is 39–87% [29–33]. Although MRI has superior soft tissue contrast resolution compared to CT, its sensitivity of 63–90% and specificity of 84–86% for diagnosis of chest wall invasion is low and similar to CT [31, 32]. Respiratory dynamic MRI may potentially improve the accuracy of this modality [33]. MRI is superior to CT in the evaluation of chest wall involvement by superior sulcus or Pancoast tumors which almost invariably invade the extrathoracic soft tissue (subclavian vessels, brachial plexus, and vertebrae) [32, 34]. Brachial plexus involvement and tumor extension into the spinal canal are contraindications to surgical resection, unlike vertebral body invasion. Invasion of the subclavian vessels, the common carotid or the vertebral artery represents a relative contraindication to surgery. These vessels may need to be ligated and CT and MRI can reveal significant atherosclerotic disease of the contralateral vessels, in which case the resection and ligation may not be feasible [35].
Nodularity of the pleura or pleural thickening associated with enhancement on CT is consistent with metastatic involvement. Thin-section CT is more sensitive in diagnosing pleural involvement [36].
In patients with bronchogenic carcinoma, CT allows accurate differentiation of potentially resectable T1–T3 lesions from unresectable T4 tumors. Mediastinal invasion cannot always be diagnosed on CT. MRI has inherent advantages including the fact that the delineation of mediastinal and hilar vessels does not require the administration of intravenous contrast medium. Increased soft-tissue characterization by MRI may be helpful in differentiating the tumor from other processes. However, MRI is costly and time consuming and its spatial resolution is inferior to that provided by CT. Although neither MRI nor CT are capable of demonstrating minimal invasion of either the mediastinal pleura or mediastinal fat, significant obliteration of fat planes or compression or encasement of vessels in the mediastinum appear to be better demonstrated by MRI than CT [3].
N Stage
The most widely used diagnostic criterion of nodal metastases is a measurement of lymph node size (short axis diameter). Other characteristics, such as shape, density, or contour do not determine nodal involvement. However, lymph node enlargement may also be due to reactive hyperplasia, and metastatic nodes are not necessarily enlarged. In a meta-analysis, CT had a sensitivity of 57%, a specificity of 82%, and positive and negative predictive values (PPV and NPV) of only 56% and 83%, respectively, for mediastinal staging [37]. CT findings can exclude more than 40% of patients from potentially curative surgery and, on the other hand, another almost 20% of patients will undergo unnecessary or non-curative surgery based on CT. CT should be seen as a tool pin-pointing to suspicious enlarged nodes for histological confirmation. MRI is not sensitive for assessment of calcifications and is therefore not routinely used in the evaluation of lymph nodes. CT and MRI have a similar accuracy for detection of mediastinal node metastases (N2 or N3) with a sensitivity of 52% and 48%, and specificity of 69% and 64%, respectively [32, 38, 39].
M Stage
Although in patients with early stage NSCLC the incidence of occult distant metastases is <1% [40], most clinicians prefer to conduct a metastatic survey in all patients [41]. The adrenals are currently evaluated by chest CT extended below the diaphragm, considering any adrenal mass with an attenuation value of <10 Hounsfield units on non-enhanced CT consistent with an adenoma [42]. If an adrenal lesion remains indeterminate on a non-enhanced CT, MRI can be helpful [42]. The liver is often included in the chest CT. If a suspicious lesion is detected dedicated CT, US, or MRI are required for its further characterization. Contrast-enhanced CT is the most commonly performed procedure in search of brain metastases in spite of the fact that MRI with contrast injection has a greater sensitivity than CT [43].
Follow-Up Imaging
After treatment lung cancer is usually followed-up by serial CT scans at 3, 6, and 12 months after completion of therapy and once a year thereafter. For patients receiving chemotherapy assessment of treatment effectiveness is crucial. CT is the method of choice for measuring lesions before and after treatment according to response evaluation criteria in solid tumors (RECIST) [44]. In patients treated with radiotherapy, CT can reveal post-radiation pneumonitis and fibrosis and monitor tumor recurrence. In patients with nonspecific CT findings suspicious for recurrent cancer, sometimes difficult to differentiate from fibrosis, MRI can be helpful [45]. Posttreatment fibrosis has low signal intensity on both T1- and T2-weighted images, whereas tumors show relatively increased intensity on T2-weighted sequences [45]. However, inflammation secondary to radiation therapy may also lead to high signal intensity on T2-weighted sequences, similar to retained secretion within traction bronchiectasis and therefore the differentiation of these two entities is not always possible.
Mediastinal Tumors
Mediastinal abnormalities are caused by a large number of processes that can involve various compartments of the mediastinum. Up to 50% of these processes are asymptomatic and discovered incidentally [46, 47]. The radiological armamentarium for evaluation of the mediastinum includes a wide variety of imaging modalities.
The standard posterior–anterior and lateral chest X-ray remains the initial diagnostic imaging tool [48]. It can reveal widening of the mediastinum, deformation of its contours, displacement of adjacent organs, and presence and type of calcification. However, its ability to delineate the extent of mediastinal processes and their relationship to specific anatomic structure is limited. This is achieved by CT which plays a crucial role in the detection and, in particular, in the characterization of mediastinal abnormalities.
Due to its excellent density resolution, CT is not only able to distinguish normal structures from pathological processes in the mediastinum, but it can also characterize the latter based on their attenuation pattern, and to accurately determine their origin and extent [49, 50]. CT resolves unclear findings on chest X-rays caused by structure superimposition. Therefore, following the detection of a mediastinal abnormality on chest X-rays, CT should be the next step in the diagnostic work-up. A second common indication for CT is the detection of mediastinal pathology which is clinically suspected but not seen on chest X-ray, such as a suspected thymoma in patients with myasthenia gravis, thymic carcinoid in patients with ectopic corticotropin production or an ectopic mediastinal parathyroid adenoma in patients with hypersecretion of parathyroid hormone [51]. CT is also one of the main imaging modalities used for percutaneous biopsy guidance.
MRI is usually reserved for cases that need further clarification of CT findings and provides additional information regarding the nature, location, and extent of mediastinal disease. Based on its ability to distinguish between different tissues, MRI can confirm the cystic nature of a lesion that appears solid on CT, it can reveal small amounts of fat, favoring the diagnosis of fat containing masses such as teratoma, hemangioma, or extramedullary hematopoiesis [52] and, in some cases it can differentiate fibrosis from viable, recurrent tumor [49, 51]. Due to its ability to image vessels, MRI is used for the investigation of mediastinal pathology suspected to be of benign vascular origin as well as for the assessment of invasion of a large artery or vein or their narrowing by a mediastinal tumor [47, 49, 53]. MRI is helpful to evaluate mediastinal abnormalities, particularly in patients in whom administration of iodinated intravenous contrast medium is contraindicated. However, MRI is limited by reduced spatial resolution as compared to that of CT and by its inability to accurately detect calcifications [49, 51, 52]. In summary, CT is the modality of choice for evaluating suspected mediastinal pathology, whereas MRI is used as a problem-solving tool. Each modality has its own particular strengths, therefore CT and MRI do not compete, but are complementary in many clinical scenarios [49, 54, 55].
Differentiating malignant from benign tumors and grading of malignancy are essential for treatment planning as well as for defining patient prognosis [56, 57]. Unfortunately, the malignant or benign nature of a lesion cannot be confirmed using imaging criteria alone. Findings of irregular contours, necrotic or cystic component, heterogeneous enhancement, lymphadenopathy, and invasion of the great vessels is strongly suggestive of a malignant thymic tumor [58], whereas a unilocular, thin-walled, water density lesion most probably represents “true” cyst [52]. Nevertheless, further investigations are often required, including noninvasive tests such as diffusion-weighted single-shot echo-planar MRI [59] or functional and metabolic nuclear medicine procedures, or invasive tests such as percutaneous or video-assisted thoracoscopic biopsy to obtain a definite diagnosis.
Thymomas, germ cell tumors, lymphomas, and neurogenic tumors account for the overwhelming majority of primary malignant mediastinal tumors in adults. Approximately 30–35% of thymomas, 20% of germ cell tumors, and 15% of nerve sheath tumors are invasive [47, 49, 50]. Radical excision is the standard of care for malignant thymic diseases and for lesions of neurogenic origin, whereas radiotherapy is the treatment of choice for seminoma. Non-seminomatous germ cell tumors are treated with a combination of surgery and chemotherapy, and a combination of chemo- and radiotherapy is administered in most cases of lymphoma [60–63].
Thymoma is the most common primary neoplasm located in the anterior mediastinum [64, 65]. It affects predominantly adults, with equal incidence in men and women, and is very rare in children [64]. Up to 50% of patients with thymoma suffer from myasthenia gravis, but some may also present with other thoracic complaints or can be entirely asymptomatic [64–66].
Thymoma is usually well defined, but it can be aggressive, penetrating the capsule and extending into the mediastinal fat, pleura, pericardium, lung, and great vessels [65, 67] and it can also cross the diaphragm, reaching the retroperitoneum [47]. The size of thymoma varies, ranging from small nodules to large masses [65]. Invasive thymoma can send drop-metastases to the ipsilateral pleura and pericardium [47]. Hematogenous metastases and lymphadenopathy are rare [65]. The presence of enlarged mediastinal lymph nodes in association with a dominant anterior mediastinal mass suggests the diagnosis of lymphoma or thymic carcinoma rather than thymoma [64, 68].
Thymoma is not always detectable on chest X-rays. Contrast-enhanced multidetector CT, with its capability for multiplanar reconstructions, is used for precise assessment of a mediastinal mass and its local invasion, aiding in the preoperative planning. Staging of thymoma is based on the widely accepted clinicopathological classification of Masoaca et al. [69] as well as on the WHO histological classification [70].
Although thymoma typically rises in the anterior–superior mediastinum, it can occur anywhere from the thoracic inlet to the cardiophrenic angle [71, 72]. Most tumors are unilateral [73], spherical or ovoid, and usually encapsulated soft tissue lesions outlined by adjacent mediastinal fat [68]. Up to 60% of thymomas demonstrate various degrees of invasion of adjacent structures [74]. The tumor generally invades vascular structures, but their encasement by neoplasm can occur as well [75, 76]. While a lack of mediastinal fat obliteration does not necessarily exclude capsular penetration, when these planes are preserved, extensive invasion is unlikely [47]. Aggressive thymoma can present with pleural involvement even at diagnosis, and in cases with circumferential lung encasement it may be indistinguishable from malignant mesothelioma [77]. While pleural metastases are common, pleural effusion is not typical for patients with thymoma [67].
Thymoma can manifest heterogeneity on CT due to cystic degeneration, hemorrhage, or necrosis [65]. Calcifications can occur, are more frequent in aggressive subtypes, and exhibit a variety of patterns ranging from curvilinear calcification of tumor capsule or fibrous septa to punctuate or coarse calcifications within the tumor itself [66, 75–78].
Thymoma has no distinctive features on MRI, which only rarely provides additional important information to an optimally performed contrast-enhanced CT study [79]. Multidetector CT scans with 3D reconstructions are at least comparable to MRI in patients with thymoma [79]. MRI can be useful in the assessment of vascular involvement in patients who cannot tolerate intravenous iodine contrast injection or for clarifying equivocal CT findings. Recent publications discuss the use of dynamic and chemical shift MRI. On dynamic MRI studies thymomas reached peak lesion enhancement earlier than other mediastinal neoplasms, earlier for lower stage tumors as compared to higher stage disease [79, 80]. Chemical shift MRI can help differentiating thymic hyperplasia from thymoma since the chemical shift ratio in thymic hyperplasia is considerably lower as compared to thymic neoplasms. This is of significance in particular in patients with myasthenia gravis, who present with a homogeneous enlarged thymus on CT and MRI which can at times be indistinguishable from thymoma [81].
On T1-weighted MR images thymoma has a similar signal intensity to muscle, while T2-weighted images show a high signal intensity. Thymomas showing a heterogeneous pattern and cystic changes present as areas of increased signal intensity on T2-weighted images [76, 81]. Hemorrhage is identified as an area of high signal intensity on both T1- and T2-weighted or on STIR images [58]. Tumor capsule and fibrous septa show low signal intensity on both T1- and T2-weighted images [58]. The role of [18F]FDG PET CT in tumors of the thymus is unclear. The majority of these lesions have SUVs <3 and a variable appearance on [18F]FDG PET. SUVs >5 suggest that the lesion may have a different etiology, such as lymphoma.
Differential Diagnosis Between Lung Carcinoma and Lymphomas
Although lymph node abnormalities depicted by CT are nonspecific, patterns of thoracic lymphadenopathy can provide important clues in the differential diagnosis of their potential etiology. Nodal metastatic spread of lung cancer always follows a similar pathway. Tumors in the right lung involve initially almost exclusively ipsilateral mediastinal nodes, while left lung tumors have a higher propensity for contralateral spread [82]. Involvement of distal nodes may occur even when proximal nodes are spared. Nearly 30% of lung cancer patients have metastases to mediastinal lymph nodes without lobar or hilar lymph nodes being involved [83]. Lack of vessel invasion by enlarged lymph nodes may help in differentiating lymphoma from metastatic lung carcinoma [84].
Lymphoma is characterized by predominantly mediastinal nodal involvement. When present, hilar node involvement is usually asymmetric and accompanied by mediastinal disease [85]. Lymphoma tends to expand along or around, rather than invade adjacent structures.
Hodgkin’s disease (HD) has a predilection for thoracic involvement. Up to 85% of patients with HD present with mediastinal lymphadenopathy, especially in the prevascular and paratracheal regions [86]. As a rule multiple sites show lymphomatous involvement although at times enlargement of a single nodal group can be encountered, usually indicating a Nodular Sclerosing histologic subtype [87]. HD typically spreads contiguously, involving adjacent lymph node groups and only rarely skips lymph node groups as described for lung cancer [87, 88]. HD also has a tendency to cause thymic involvement in association with mediastinal lymph node enlargement [89].
Thoracic disease is less common in non-Hodgkin’s lymphoma (NHL) and is found in up to 50% of cases [90]. The pattern of nodal involvement is also somewhat different from other types of malignancy. Single site lymph node enlargement is much more common in patients with NHL and occurs mainly in posterior or superior mediastinal or anterior diaphragmatic nodes [91]. Hilar node involvement is less common than for HD or lung cancer. Extranodal disease frequently presents with NHL and may involve the lungs, pleura, pericardium, and chest wall [90].
Sarcoidosis
Approximately 70% of patients with sarcoidosis present with a characteristic radiologic pattern including hilar and mediastinal lymphadenopathy with or without concomitant parenchymal abnormalities [92–94]. However, in up to one-third of cases radiological findings are nonspecific. The most frequent presentation is that of right paratracheal and bilateral hilar lymphadenopathy. The aorto-pulmonary, subcarinal, and retroazygous lymph nodes may be enlarged as well [95]. The posterior mediastinum is less commonly involved. Isolated lymphadenopathy in the anterior or posterior mediastinal compartments as well as unilateral hilar disease is more suggestive of lymphoma, metastatic lung cancer, or a granulomatous or infectious process rather than sarcoidosis. Lymph node calcification (occasionally with an eggshell pattern) can be found in up 25% of cases [95].
Parenchymal sarcoid can show a variety of radiographic patterns, including fine nodular, reticulonodular, or acinar lesions (poorly marginated, small to large nodules or coalescent opacities), and only rarely focal lesions. Acinar opacities or interstitial granulomas may coalesce to give the appearance of the alveolar form of sarcoidosis, and an air bronchogram may be detected [96]. High-resolution CT (HRCT) findings include areas of ground-glass attenuation, subpleural or perivascular nodules, which appear as beading and irregular thickening of bronchovascular bundles and thickening of interlobular septa. The nodules, corresponding to coalescent interstitial granulomas, have irregular margins. The foci of ground-glass attenuation may represent areas of active alveolitis or diffuse microscopic interstitial granulomas [97–101]. Patients older than 50 years have a higher prevalence of solitary and multiple mass-like opacities in the lung at presentation [101]. In the presence of cavitation of parenchymal lesions, which occurs in less than 1% of patients with sarcoid, tuberculosis, and fungal infections should be ruled out.
Histoplasmosis
Radiographic findings of thoracic histoplasmosis depend primarily on the clinical type of presentation and on the immune status of the host. An SPN is common in patients with an asymptomatic primary infection. The size of such nodules varies from a few millimeters to several centimeters. Most have well-defined margins and central, laminar, or diffuse calcification patterns. In some cases these nodules are slow growing, and in such cases histoplasmosis may be difficult to distinguish from malignancy.
Adenopathy is frequently seen in addition to parenchymal lung abnormalities, often with calcifications. The differential diagnosis of non-calcified mediastinal adenopathy includes sarcoidosis, lymphoma, and metastases. Enlarged lymph nodes may cause significant bronchial or tracheal compression or obstruction and may lead to esophageal obstruction as well [102].
In more severe cases, radiographs may show widely disseminated, diffuse, fairly discrete nodular shadows throughout the lungs, with individual lesions measuring 1–10 mm diameter. This form of disease is known as miliary histoplasmosis, and appearances are similar to miliary tuberculosis [103]. The infiltrates clear within 2–8 months; however, fibrotic lesions may calcify and persist for many years.
Lung cavitation is usually noted in patients with underlying obstructive lung disease and is similar to chronic active tuberculosis, with predominantly upper lobe disease, characterized by fibrosis, necrosis, cavitation, and granulomatous inflammation. Fibrosing mediastinitis can develop in some patients. It is important to define the extent of the fibrous mass in the mediastinum or hilum and to identify if it causes obstruction of the superior vena cava, pulmonary vessels, esophagus, trachea, or bronchi. Stippled or dense calcifications within the mass are present in most patients with fibrosing mediastinitis [104].
Coccidioidomycosis
Thoracic manifestations of primary coccidioidomycosis include parenchymal disease, lymphadenopathy, and pleural effusion [105]. Parenchymal consolidation, single or multiple, is the most common manifestation found in 75% of patients. It is segmental or subsegmental, usually unilateral and with a perihilar or basal distribution. It can resolve spontaneously within 1–2 weeks. Nodular lung disease is found in up to 20% of patients. The nodules are frequently well defined, simulating metastases, but may also present with ill-defined margins. They are 5–25 mm in diameter and have a parahilar and lower-lobe distribution. In approximately 20% of patients, hilar adenopathy is also present, usually unilateral and concomitant with parenchymal lesions. Mediastinal adenopathy is seen in the presence of severe and prolonged infection and is associated with a higher risk of dissemination. Pleural effusion occurs in less than 20% of patients, although pleuritic chest pain is more frequent, in 50–75% of cases [105].
Approximately 5% of patients may develop a persistent pulmonary disease such as pneumonia with or without adenopathy, nodules and cavities, bronchiectasis, empyema, or calcifications. Pulmonary nodules are the most common radiographic findings in persistent pulmonary coccidioidomycosis infection. They are, as a rule, single, well circumscribed and round, averaging 1.5–2 cm in diameter, and tend to occur in the periphery of the middle and upper lobes of the lungs, in contrast to tuberculosis, when nodules develop mainly in the anterior segment of an upper lobe. Coccidioidomycosis related nodules can remain stable for months and eventually regress. Only rarely is slow growth observed [106].
Calcification is much less frequent in coccidioidomas than for tuberculosis and histoplasmosis. Therefore in the evaluation of these nodules, malignancy is a primary concern for the clinician. Cavitation may develop as a result of necrosis in an area of pneumonia or may be the result of the excavation of a nodule. Usually cavities appear as a single lesion, are located in the upper lobes, and can have thin or thick walls. Thin-walled cavities have a tendency to change in size, possibly reflecting check-valve communication with the bronchial tree. A rapid change in size of a cavity suggests coccidioidal infection rather than any other granulomatous process.
Disseminated coccidioidomycosis may occur as a complication of the primary illness, a late complication of chronic disease, or may represent the reactivation of latent disease in susceptible individuals. Dissemination has a miliary pattern that resembles tuberculosis, although with less well-defined nodules. The differential diagnoses of this pattern also includes other mycotic infections, silicosis, sarcoid, and metastatic disease. Hilar and mediastinal adenopathy is almost always associated with disseminated disease [106].
Tuberculosis
Primary lung tuberculosis has a nonspecific imaging presentation [107–110]. Common findings include segmental or lobar airspace consolidation, ipsilateral hilar and mediastinal lymphadenopathy, and/or pleural effusion.
Airspace consolidations tend to be homogeneous, with ill-defined margins and usually occur in the lower and middle lobes, and in the anterior segments of the upper lobes. Cavitation within parenchymal opacity is uncommon in primary infection. The lung opacity tends to become rounded with healing and continues to shrink until only a small nodule remains. Subsequently, the nodule may become calcified or ossified, resulting in a calcified granuloma.
Lymphadenopathy is a common manifestation of primary pulmonary tuberculosis. The presence of hilar and mediastinal lymphadenopathy helps in differentiating primary from postprimary tuberculosis where it is conspicuously absent. Lymphadenopathy may occur as the only manifestation of primary pulmonary tuberculosis, without a concomitant parenchymal opacity. This is more common in patients with HIV infection. As expected, adenopathy is mainly found in the ipsilateral hilar region. Hilar lymphadenopathy is seen in approximately 60% of children with primary tuberculosis, paratracheal adenopathy is found in 40%, and subcarinal lymphadenopathy in 80% of pediatric patients. In adults, lymphadenopathy is unusual in an immunocompetent host but is more frequent in blacks and Asians. Adenopathy is common in patients with an HIV infection. On CT following contrast injection involved lymph nodes demonstrate central hypoattenuation with peripheral rim enhancement. Sometimes the pattern of lymphadenopathy is indistinguishable from that of sarcoid or lymphoma. With an appropriate immune response or with adequate chemotherapy, enlarged necrotic lymph nodes may diminish in size and commonly calcify. Calcified lymph nodes and a granuloma represent the Ranke complex.
Airway involvement is frequently present in primary tuberculosis due to bronchi compression by enlarged lymph nodes or endobronchial spread of infection, broncholithiasis, or bronchiectasis.
Parenchymal abnormalities of postprimary tuberculosis show a wide spectrum of findings [111], including patchy or confluent airspace opacities in the apical and posterior segments of the upper lobes and the superior segments of the lower lobes, cavities with a thick outer wall and a smooth inner contour and air–fluid levels [112], tuberculomas appearing as discrete, sometimes calcified, nodules, typically within the upper lobes, widespread ill-defined acinar shadows manifesting endobronchial spread, military (hematogenous) spread, appearing as circumscribed nodules less than 1–2 mm in diameter located diffusely throughout both lungs. In contrast to primary tuberculosis, lymphadenopathy is notably absent in patients with postprimary tuberculosis, with the exception of patients with HIV/AIDS infection. Bronchiectasis may occur as well, as a consequence of fibrosis. Pleural involvement is more common in postprimary tuberculosis than in primary infection, including empyema or empyema necessitans.
PET and PET/CT for Evaluation of Lung Cancer
[18F]FDG PET/CT has been used extensively in the differential diagnosis and characterization of single pulmonary lesions. While the overall performance of [18F]FDG imaging for diagnosis of pulmonary malignancy is good there are pitfalls and limitations related to potential false-positive and false-negative findings. Inflammatory lesions such as sarcoid or tuberculosis can take up [18F]FDG. While the pattern, and at times, intensity of uptake may be helpful to appropriately identify the etiology of the lesions, biopsy may be necessary to make a definitive diagnosis. False negatives may be due to small size lesions or to the histological subtype of well-differentiated malignancies such as bronchiolo-alveolar carcinoma (BAC) or carcinoid [113], which often exhibit low glucose utilization rates and low-level metabolic activity and have therefore low or absent [18F]FDG uptake. These are slow-growing tumors with less proliferative potential and longer mean doubling times as compared to NSCLC. The sensitivity of [18F]FDG imaging for BAC is only around 50% [114]. On the other hand, multifocal BAC appearing as multiple nodules or ground glass opacities (GGOs) is detected with a high sensitivity by [18F]FDG imaging studies [115].
In spite of the above-mentioned limitations, [18F]FDG PET/CT changes management in 25–52% of patients with NSCLC and has a major role in reducing the number of futile thoracotomies [116, 117]. Pretreatment [18F]FDG PET/CT provides prognostic information. Pillot et al. have summarized the literature assessing the relationship between the SUV of the tumor and outcome [118], suggesting that the SUV is a powerful surrogate marker for outcome in NSCLC. Recently, Goodgame et al. retrospectively analyzed 136 patients with stage I NSCLC. Thirty-two patients had recurrence during a median follow-up of 46 months. In multivariate analysis, a preoperative SUVmax of 5.5 or higher was an independent predictor of relapse and death in this group of patients [119]. Tann et al. also conducted a retrospective study in 51 patients with stage I lung cancer, comparing [18F][18F]FDG PET/CT results to growth rates and tumor doubling times obtained from pretreatment chest CT examinations performed more than 25 days apart. Rapid, moderate and slow doubling times correlated with SUV measurements [120]. If these preliminary results will be proven in large prospective trials, a single pretreatment [18F]FDG PET/CT study may predict in future the rate of tumor growth and be used in an individualized approach to therapy.
Since most lung lesions are hyper metabolic (with the exception of bronchoalveolar carcinoma) [18F]FDG PET-CT imaging can provide important information for the management of patients with lung lesions. [18F]FDG PET CT is recommended as a component of initial evaluation in T1–T3 disease. The imaging findings can play a pivotal role in determining whether T1 disease is resectable or whether patients with more advanced disease have metastases that may require specific therapy. In addition to defining whether pulmonary nodules represent lung cancer, [18F]FDG PET CT can determine the number and location of hypermetabolic lesions in the chest and elsewhere and define tumor response to therapy.
A baseline study in patients with pulmonary nodules can help distinguish benign from malignant disease. This is usually accomplished by evaluating a combination of the appearance of the lesion on CT, SUV of lesion (values >2 are suspicious and values >4 require further evaluation, usually with a biopsy), and detect other sites of hypermetabolism. If there are multiple hypermetabolic foci in addition to the nodule under investigation, the lesion is more likely to be neoplastic than inflammatory in etiology.
A baseline study in a patient with histologic evidence of lung cancer should evaluate the lung parenchyma, bronchus, lymph nodes (in the chest, neck, and abdomen), chest wall, pleura, liver, adrenal glands, and bone for hypermetabolic disease to stage the disease, and address the question about the potential roles of surgery, radiotherapy, and chemotherapy in patient management. Approximately 30% of NSCLC patients are candidates for curative resection.
Serial scans are helpful for surveillance, to detect recurrence, especially in patients with no clinical evidence of disease, and to determine the effectiveness of therapy, by detecting changes in lesion size and SUV, as well as detecting new lesions.
Characterization of the Solitary Pulmonary Nodule
A single pulmonary nodule is seen as an opacity in the lung parenchyma measuring <3 cm (larger lesions are masses) without associated adenopathy or atelectasis [121]. Lung nodules can be caused by infection, inflammation, and neoplasms (about 80 different etiologies have been identified). CT alone, or with the addition of [18F]FDG PET-CT, allow the majority of pulmonary nodules to be categorized as benign or malignant. Lesions without spicules, with uniformly distributed dense calcification (>300 Hounsfield Units, including the center of the nodule) and which have <15 HU enhancement with contrast administration are radiographically benign. On the other hand, spiculated borders, indistinct margins, extension to pulmonary veins, focal retraction of adjacent pleura, heterogeneous composition, or enhancement of >25 HU following injection of intravenous contrast, suggest malignancy. However, even after careful assessment by CT, distinguishing a malignant from a benign lesion is difficult. Lesions <7 mm diameter have <1% likelihood of malignancy [122]. Lesions 7–20 mm have a 15% incidence of malignancy, and lesions >20 mm have an 81% likelihood of malignancy. Determining the metabolic status of lesions >0.7 cm in diameter with [18F]FDG PET-CT can determine if these lesions are malignant or benign in ∼90% of lesions. Lesions with SUV ≤2.0 have a low likelihood of malignancy, reducing the need for biopsy, lesions with SUV >4.0 are more likely malignant, and lesions between SUV 2.0 and SUV 4.0 are indeterminate. Nevertheless, an [18F]FDG-negative lesion in a patient with high clinical suspicion for malignancy (e.g., history of smoking, exposure to asbestos, older age, and/or history of non-thoracic neoplasm) still needs to be biopsied to establish the etiology of the lesion. In the presence of a low clinical suspicion in a patient who is at high risk for biopsy, follow up by further imaging is usually indicated. If patients carry a high clinical risk, biopsy, and histologic evaluation of the lesion is advisable if at all possible. While it could be argued that [18F]FDG imaging is not indicated for diagnostic purposes in patients with a high clinical suspicion (as they would need biopsy or surgery), its main role in the management of these patients is to determine if disease is localized prior to possible surgical or radiation therapy.
In a meta-analysis of 1,474 pulmonary lesions of any size, Gould et al. [123] found a sensitivity of 96.8% and specificity of 77.8% for [18F]FDG-PET to distinguish malignant from benign etiologies. In another meta-analysis including five prospective studies, Hellwig et al. [124] reported a sensitivity of 93% and specificity of 87% and PPV and NPV of 94% and 89%, respectively. The risk to miss malignancy was 11%. Yi et al. [125] showed that [18F]FDG PET/CT is more accurate than helical dynamic CT for SPN characterization and therefore, where available, should be performed as a first-line evaluation. The semiquantitative criterion of a mean SUV ≥ 2.5 has been shown prospectively to have a sensitivity of 90–100% and specificity of 69–95% for diagnosis of malignancy [126–128] (Fig. 13.2). In a prospective study in 585 patients, Bryant et al. [129] demonstrated that in nodules less than 2.5 cm in diameter an SUVmax of 2.5 or less was associated in 24% of cases with malignancy, as compared to lesions with an SUVmax of 2.6–4 which have an 80% chance of malignancy, and lesions with an SUV above 4.1 with a 96% probability. In spite of these data, Hashimoto et al. [130] have recently shown that in SPNs with an SUVmax < 2.5 visual analysis performs as well as semiquantitative measurements. The probability of malignancy in a visually evident lesion was 60%, but decreased significantly in lesions with no [18F]FDG uptake.
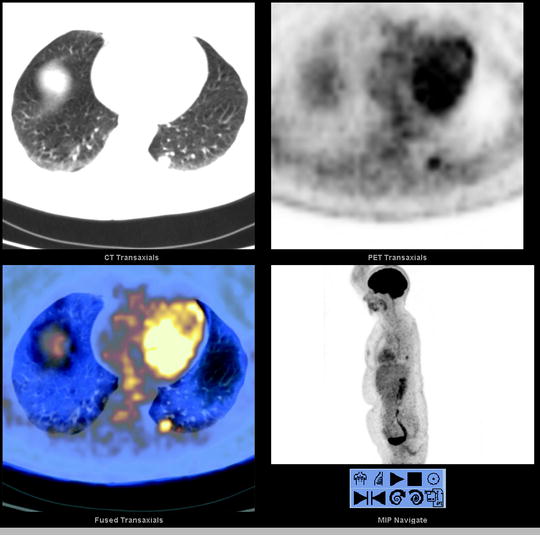

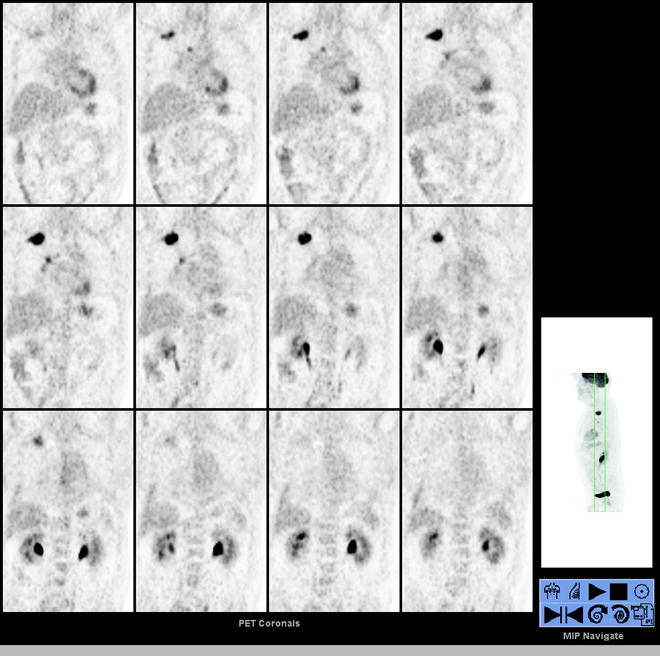
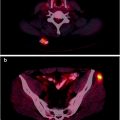
Fig. 13.2
A 52-year-old woman with a 1.4-cm LLL nodule was referred to PET/CT for presurgical assessment. Selected transaxial CT, PET, and fused PET/CT slice and MIP image (lower right) show the [18F]FDG avid LLL nodule (SUVmax 4.4). There is no hilar nor mediastinal [18F]FDG avid lymphadenopathy and no distant [18F]FDG avid disease, corresponding to T1aN0M0 disease by PET/CT. LLL left lower lobe, MIP maximum intensity projection
Location
The question of the location of the lesion is usually well answered by CT or the CT component of PET/CT. The exact location of the tumor and its anatomical relationship to important structures such as the bronchi, pleura, pulmonary veins, and thoracic aorta, as well as defining the presence of mediastinal or chest wall invasion, are important in the decision-making process for further management.
Size
The question of the size of the lesion is also answered well by CT in most patients. However, in the presence of atelectasis distal to the tumor, the differential diagnosis between the tumor mass and the area of atelectasis may be difficult to determine on CT. The [18F]FDG study can define which portion of the lesion is associated with metabolically active tumor, providing additional valuable information for staging. Precise measurement of tumor size is required for TNM classification (Table 13.2) [121]. Subclassification according to tumor size is a factor that has an impact on prognosis (Table 13.3) [23]. In addition, since in most patients there is an interval of few days to a few weeks between the initial staging CT and the [18F]FDG study, exact measurement of the tumor size on the CT component of PET/CT study can help the clinician in determining the growth rate of the tumor.
Solitary Pulmonary Nodule
[18F]FDG imaging has an established role in the evaluation of SPNs to differentiate benign from malignant lesions. An SPN is usually defined as any nodule up to 3 cm in diameter [121]. The majority of SPNs (60–70%) are benign in etiology. Lung cancer, however, is the etiology in 20–30% of SPNs. [18F]FDG imaging is a better predictor of malignancy in SPN than a combination of clinical and morphologic conventional imaging criteria [114, 131].
False-negative [18F]FDG PET/CT (no uptake in a malignant SPN) is found in less than 5% of nodules 0.6–3 cm in diameter [20]. In the NY-ELCAP study, 378 nodules less than 5 mm in diameter were all nonmalignant on histopathology, therefore suggesting the need for further follow up only in nodules that are 5 mm in diameter or larger [132]. In nodules 5–10 mm in diameter, Bastarikka et al. [133] report a sensitivity of 69%, which increased to 95% in nodules of greater than 10 mm for the detection of malignancy by [18F]FDG imaging. The authors also noted that uptake decreased when the size of the nodule was less than twice the system resolution (7–8 mm), and therefore different criteria may need to be applied for nodules less than 15 mm in diameter.
False-negative [18F]FDG imaging results are usually due to the small size (less than 1 cm) of the SPN or a lesion histology indicating BAC or carcinoid. In a series of 36 patients with bronchoalveolar cell carcinoma, 18 patients (50%) had SUVmax ≤ 2.5 [134].
As for NSCLC, tumor [18F]FDG avidity on PET predicts mortality in patients with BAC [134]. In NSCLC patients, an SUVmax of the primary tumor >7 [135] and >10 [136] had an independent prognostic impact. Few other series also found that the SUVmax was an independent predictor of disease free and overall survival [137, 138]. Ahuja et al. [136] found that the median survival decreased with increasing mean SUV levels in 155 patients with NSCLC. In a retrospective study in 100 patients, Downey et al. [139] showed that the 2-year survival rates were 68% for patients with an SUVmax above 9 and 96% in patients with SUVmax below nine. In patients with NSCLC, Cerfolio et al. [140] found the SUVmax to be an independent predictor of tumor aggressiveness, and a more accurate predictor of tumor recurrence and survival as compared to TNM staging.
Over 40% of SPNs are granulomas, including mycobacterial infection or histoplasmosis. The average SUVmax was 5.05 in patients with pulmonary mycobacterial infections (lesions >2 cm). Therapy resulted in a reduction of [18F]FDG uptake to near negligible levels [141]. [18F]FDG PET/CT has a promising role for evaluation of TB in high-risk immunocompromised patients with cancer [142]. In an area with endemic histoplasmosis, inflammatory lesions were difficult to separate from non-small cell lung cancer (mean SUVmax 3.2 in benign lesions and 8.5 in neoplastic lesions with significant overlap between groups [143]).
Nodal involvement with sarcoidosis can also be a confounding factor in evaluating the performance of [18F]FDG imaging in SPNs. In one series, 95% of patients with sarcoidosis had pulmonary nodal uptake (SUV 8.6) and 66% of patients had parenchymal uptake (SUV 4.7) [144]. In cancer patients with biopsy-proven sarcoidosis early metabolic response to systemic steroid treatment can help in the final differential diagnosis between cancer and sarcoid when both coexist [145]. Other inflammatory conditions such as pneumonia, pyogenic abscess, aspergillosis, Wegener’s granulomatosis, and anthracosis may also need to be differentiated from lung cancer. In an [18F]FDG-avid SPN and low clinical suspicion of malignancy, further investigation is indicated to determine the cause of tracer uptake.
Dual-phase [18F]FDG studies at 1 and 2 h after tracer injection have been suggested to increase the sensitivity and specificity of [18F]FDG imaging [146, 147]. Using an SUV cut-off of 2.5 and a 10% increase in SUV as a threshold of malignancy, Matthies et al. showed an improvement in sensitivity from 89 to 100%, associated however with a deterioration in specificity from 94 to 89% in single- and dual-phase PET studies, respectively, possibly due to differences in glucose-6 phosphatase and hexokinase levels within benign and malignant cells [146]. However, active granulomas, a frequent cause for false-positive [18F]FDG avidity, may also demonstrate the same increase in tracer uptake over time [131].
Other Lesions
[18F]FDG PET-CT imaging can identify incidental lesions or entities such as inflammatory processes. However, it may be difficult to distinguish inflammation secondary to radiation therapy or recent interventional procedures from disease persistence or recurrence, especially if serial [18F]FDG scans are used for surveillance.
[18F]FDG Imaging for Preoperative Staging of NSCLC
Accurate staging of NSCLC provides important prognostic information and determines the best treatment approach.
T Stage
N Stage
Based on the criterion that a node of 10 mm or more in the short axis diameter is involved with metastatic disease, the sensitivity of CT ranges between 52 and 69% and the specificity between 69 and 82% [152]. Normal size lymph nodes on CT have micrometastases in 15% of cases [153], while 30–40% of enlarged lymph nodes have no tumor cells [154]. [18F]FDG imaging, on the other hand, was found to have a sensitivity ranging between 79 and 85%, specificity of 89–92% [155, 156], and an NPV above 90% [157] (Figs. 13.3 and 13.4) for the detection of metastatic mediastinal lymph node involvement [151]. False-negative results can occur in cases of micro-metastases and false-positive findings in cases of inflammation. The PPV for evaluating the mediastinum ranges from 45 to 93% [158–160]. Mediastinoscopy is therefore the standard-of-care for mediastinal staging. In cases of [18F]FDG-positive mediastinal nodes PET can further direct biopsy. Antoch et al. [15] assessed 27 NSCLC patients with CT, [18F]FDG-PET, and PET/CT with histopathology: PET/CT classified the tumor stage correctly in 26 patients compared to 19 with CT and 20 with PET. The accuracy for regional lymph node staging was 93% with PET/CT, 89% with PET, and 63% with CT. PET/CT also detected 17 distant metastases in 4 patients, compared to 14 metastases in 4 patients with CT and only 4 in 2 patients with PET [15]. Shim et al. prospectively recruited 106 patients with NSCLC who underwent tumor resection and lymph node dissection after CT and [18F]FDG-PET/CT. PET/CT correctly staged 86% of the primary tumors as compared to 79% by CT. PET/CT was more sensitive and more specific as compared to CT (85% vs. 70% and 84% vs. 69%, respectively) for diagnosis of malignant regional nodes, with more false-positive findings and false-negative findings on CT compared to PET/CT [150].