Fig. 9.1
A low frequency probe is normally used for depth imaging but has less resolution compared to a high frequency probe. High frequency probes are optimal for imaging relatively superficial structures like the pleura
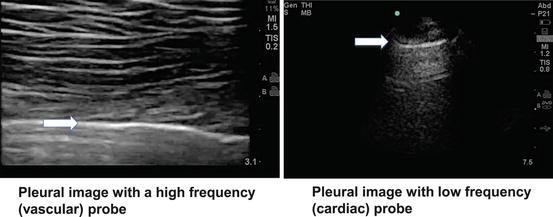
Fig. 9.2
Contrast of pleural images obtained using a high frequency versus low frequency probe. Arrow indicated hyperechoic pleural line
ICU patients frequently present challenges regarding proper positioning for clinical tasks, which can be due to many factors such as external devices (e.g., intra-aortic balloon pump, intravenous lines, monitor leads, endotracheal tubes with mechanical ventilator support), patient body habitus, and hemodynamic instability. However, lung ultrasound can be adapted to the patient, and can be performed with the patient sitting up or in the supine position. In mechanically ventilated patients, the only possible way to scan the chest is often in a supine or semi-recumbent position. If a patient is able to maintain an upright position, the posterior aspect of the chest can be readily scanned. When scanning the axillary area of the chest, the patient’s arm on the ipsilateral side can be abducted away from the patient’s chest or moved across the body to increase the viewing area. This maneuver also can be performed in intubated patients provided that there is no restriction of movement (e.g., arm injury, arm restraints/sling).
Patience is key in obtaining images using ultrasound. Oftentimes, minute movements of the probe are necessary to improve image quality. Increasing gain does not automatically improve the image caliber but may even worsen image quality by increasing noise. Caution should also be observed when applying excessive pressure on the chest with the probe since this may not improve image quality, but may inflict pain on the patient.
The probe must sometimes be placed on the most posterior part of the thorax to scan for consolidation, atelectasis, or pleural effusion in intubated patients. While this can be uncomfortable for patient and operator, since these patients are typically supine these findings are more likely to be found in the most dependent part of the chest. A complete examination of the recumbent patient therefore necessitates posterior scanning.
Image Acquisition and Interpretation
Lung ultrasound can be performed with the patient in a variety of positions, and as such, approaching the patient systematically to ensure thorough scanning of the entire thorax is important. The ultrasound transducer should be held like a pen to allow for the ultrasound beam to be manipulated readily in all planes. The probe marker should be oriented cephalad, and the marker dot should be oriented on the left side of the ultrasound screen (i.e., the left side of the screen should correspond to the cephalad part of the patient). The transducer is placed in between the ribs and angled medially and laterally in a rib interspace, continuing to the next interspace from cephalad to caudad (Fig. 9.3). Subtle changes in the rotation, tilt, and angle of the probe are helpful in obtaining quality images – especially underneath the rib since bone prevents ultrasound transmission.
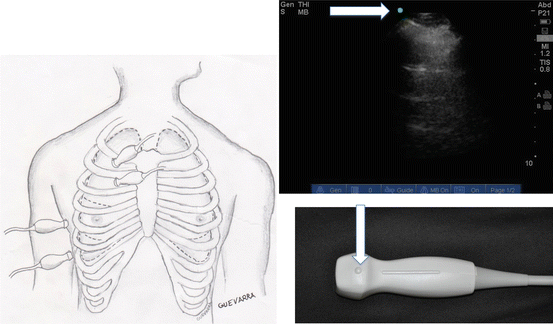

Fig. 9.3
Diagram of ultrasound scanning approach. Note that the transducer is placed in between the ribs. The arrows correspond to the marker on the screen and on the transducer, which should normally be oriented cephalad
In order to thoroughly scan a patient’s thorax, the chest wall is divided into four zones to ensure systematic scanning. Zone 1 corresponds to the anterior part of the chest, zone 2 to the lateral part of the chest, and zone 3 to the posterior and most dependent part of the chest. [1] Zone 4 (not shown) corresponds to the postero-medial part of the thorax, which can be scanned if the supine patient is placed in lateral decubitus position (Fig. 9.4). Scanning the thorax completely is important, especially including the most basal portion of the thorax bringing images of the liver or spleen into view. Finally, understand that the nature of the ICU patient population often presents limitations to obtaining adequate lung ultrasound images for various reasons; these are listed in Table 9.1.
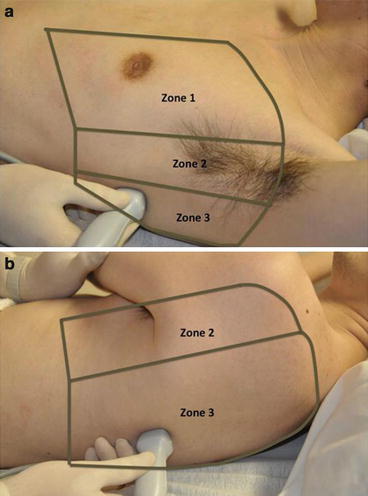

Fig. 9.4
(a) Ultrasound scanning zones (anterior view). (b) Ultrasound scanning zones (posterior view). Zone 4 not shown
Morbid obesity with excessive pannus |
Subcutaneous emphysema |
Cachexia (unable to place U/S probe in the rib interspace) |
Chest wall dressings |
Lines, tubings, and electrocardiogram leads |
Inability to properly position the patient |
Inability to place ultrasound probe in the posterior aspect of the chest |
Pneumothorax |
Shotgun pellets |
Pleural calcifications |
Basic Competencies
Normal Lung and A-Lines
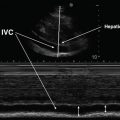
As is the case with many facets of medicine, a firm grasp of “normal” is important – and the image of the normal lung under ultrasound is no exception. Air prevents ultrasound wave transmission, while fluid promotes it. Therefore, ultrasound waves cannot transmit through organs that are gas-filled, and the waves that are reflected in gas-filled organs, such as the lung, produce amorphous gray images on the screen. Bones, such as the ribs, do not transmit ultrasound waves and appear as anechoic “shadows.” A normally aerated lung ultrasound is depicted in Fig. 9.5, showing first the hyperechoic pleural line, followed by amorphous gray parenchyma (due to lung being filled with air). The anechoic black images running longitudinally on the right side of the image are the normal appearance of rib shadows. Finally, the regularly spaced echogenic horizontal lines (termed “A-lines”) are produced as reverberation artifacts reflecting the sliding pleural line and appear at multiples of the distance from the body surface to the pleural line, appearing to project into the air-filled lung [3].
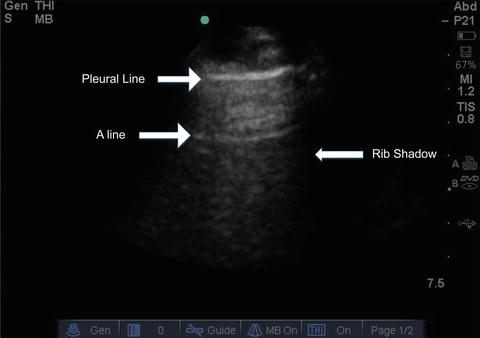

Fig. 9.5
Ultrasound image of a normal fully aerated lung. The black veil-like structure that borders the lateral segments of the image corresponds to the rib shadow resulting from reflection of the ultrasound beam. An A-line corresponding to a reverberation artifact is indicated. Repeated A-lines may be seen at multiples of the distance to the pleural line
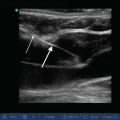
B-Lines
An interruption or alteration in the normal aeration of the lung leads to abnormal findings on lung ultrasound. The presence of fluid or other material in the lung interstitium can be determined using ultrasonography. This can be seen as an increased number of vertical artifacts arising from the pleural line called “B-lines” (also referred to as “comet tails” or as “lung rockets” if several are present in a group) (Fig. 9.6; also see, e.g., Figs. 14.25 and 15.33) [3]. B-lines begin at the pleural line as a narrow hyperechoic beam that broadens as it goes deeper, extends the entire depth of the image, and effaces the normally appearing A-lines (since there is a loss of normal aeration of the lung) [4]. The intensity of B-lines increases with inspiratory movements. Random B-lines can be a normal finding at the lung bases due to small areas of atelectasis in that region [5]. Therefore, when scanning for pathologic B-lines or B-lines that have clinical relevance, start scanning at the least-dependent part of the thorax first. In other words, start the scan at the apical part of the thorax in an upright patient, or at the most anterior part of the chest in a supine patient. The variations in the number and spacing of B-lines can be used in conjunction with other findings to provide diagnostic relevance, as discussed in the sections below. Z-lines should not be confused with B-lines – these are comet-tail-like artifact that do not extend the entire depth of the imaging, are not as echoic as the pleural line, and do not efface the A-lines [3]. They are of uncertain clinical significance and may be present in healthy individuals [3].
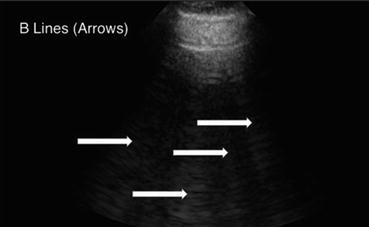

Fig. 9.6
B-Lines (white arrows) in a patient with pulmonary edema. In the presence of B-lines, A-lines will be abolished
Lung Sliding and Lung Pulse
A fully inflated lung, or more specifically the pleural surface of the inflated lung in contact with the chest wall, can be visualized through ultrasonography. The pleura appears as a hyperechoic shimmering linear structure (pleural line) that moves back and forth in concordance to the respiratory cycle (called “lung sliding”) [6] or the cardiac cycle (called “lung pulse”) [7]. Lung sliding can be absent in the setting of pneumothorax, but also with severe hyperinflation resulting in impaired diaphragm excursion and poor lung ventilation. The presence of a lung pulse, not normally visualized when ventilation is normal, indicates that pneumothorax is not present, as the cardiac pulsation is being transmitted through the lung to the pleural surface. These findings are further discussed in the chapter on pleural ultrasound, but the presence or absence of these findings is important in the complete interpretation of lung ultrasound for respiratory failure.
Alveolar-Interstitial Syndrome
As stated earlier, normal lung is air-filled with a very small amount of fluid. Air has different acoustic impedance characteristics compared to fluid, and the image produced is different once there is alteration of the air-fluid interface of the lung interstitium (e.g., in cases such as pulmonary edema or inflammation). B-lines or comet tail artifacts are seen when a small water-rich structure, such as an edematous interlobular septa, is surrounded by air, resulting in a high impedance gradient. It is absent under normal conditions and present in alveolar-interstitial syndromes [8]. The alveolar-interstitial syndromes refer to a collection of disorders that result in interstitial and/or alveolar fluid accumulation producing increased B-lines on lung ultrasound and resulting in the impairment of the alveolocapillary gas exchange capacity of the lungs and acute, subacute, or chronic respiratory failure. Examples include acute conditions, such as congestive heart failure, acute respiratory distress syndrome, or other causes of pulmonary edema, and chronic conditions such as pulmonary fibrosis [8]. The alveolar-interstitial syndrome is therefore not a diagnosis itself, and the clinician must distinguish the underlying cause of the alveolar-interstitial process. B-lines arising from alveolar-interstitial syndrome with thickening of interlobular septa appear as regularly spaced B-lines, on average a distance of 7 mm apart. B-lines that are positioned closer to each other (generally less than 3 mm), are more typically seen in a generalized alveolar-filling process or in the setting of subpleural ground glass lesions [8]. More irregularly spaced B-lines are from disseminated foci of pneumonia [2]. B-lines are more clinically relevant if there are more than three B-lines per single field and they present all over the surface of the lung, especially in the most apical portion, or in the anterior part of the thorax in a supine patient [2, 8, 9]. Presence of B-lines, in addition to the presence of lung sliding, also rules out the possibility of pneumothorax when assessing for the cause of respiratory distress [10, 11].
Pneumonia and Consolidation
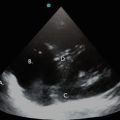
Pneumonia is a clinical diagnosis supplemented by radiographic findings. It is sometimes difficult to make the diagnosis when the symptoms and/or the radiographic findings are vague, such as in the setting of faint interstitial findings or retrocardiac opacities on chest radiography. Lung ultrasonography can help elucidate lung pathology that otherwise can be difficult to interpret on standard radiographic testing. In general, consolidated lung can be visualized via ultrasonography due to the loss of normal lung aeration, and will appear similar to other “tissue” images (similar to the liver, i.e., “hepatization” or to the spleen) [2]. A consolidated lung with persistent aeration of the airways will have the added appearance of hyperechoic small round structures – representing sonographic air bronchograms (Fig. 9.7). The consolidated lung has an irregular border delineated by a normal lung or by an effusion. This is called the “shred sign,” and the asymmetric border is called the “shred line” [1]. If the consolidated lung moves with the respiratory cycle, it is likely the airway leading towards the affected segment is nonobstructed [2].
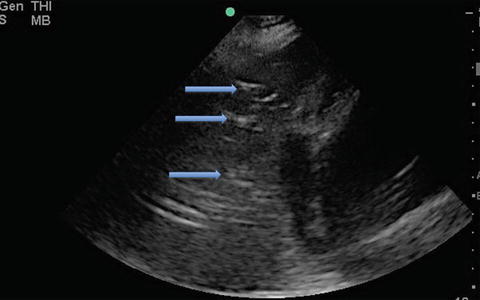

Fig. 9.7




Ultrasound image of PLAPS – posterolateral alveolar and/or pleural syndrome. The hyperechoic white specks (blue arrows) in the consolidated lung are sonographic air bronchograms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree