Fig. 1
a SEM images after the labeling procedure: left MAA, right HSA spheres, ultrastructural analysis (Maus et al. 2011). b Stability tests in sterile water, 0.9% sodium chloride (NaCl), and human plasma were performed (Sect. 2.2.5). No significant release of Ga-68 could be detected during the measurement period of 240 min
MAA used for pulmonary perfusion scintigraphy contained approximately 2 × 106 microspheres with particle sizes between 3 and 150 μm. However, approximately 95% particles were specified within the range of 3–40 μm. HSA spheres, also used for perfusion diagnostics, contained 3–5 × 105 microspheres in the narrower size range of 10–30 μm (Maus et al. 2011). Structural analyses of MAA and HSA by SEM showed no modification of the particles due to the labeling process (Fig. 1a). In addition, in vitro experiments showed stability of Ga-68-labeled MAA in sterile water, 0.9% NaCl, and human plasma. No significant release of Ga-68 could be detected during the measurement period (Fig. 1b).
3.2 Exploratory Studies
3.2.1 Animal Study
MAA and HSA particles were completely retained in the lung of the animals as demonstrated by the animal PET analyses (Fig. 2a). No significant differences in retention in the lung could be found. The time–activity curve indicated high in vivo stability, since no decrease of activity or migration of particles was observed within the first hour (Fig. 2b). No significant retention of Ga-68 MAA or Ga-68 HSA particles in the liver was detected (Maus et al. 2011). Additionally, we could demonstrate complete incorporation of Ga-68-labeled carbon particles (Galligas) in the lung of a Sprague–Dawley rat without any extrapulmonary activity being visualized (Fig. 2c, d).
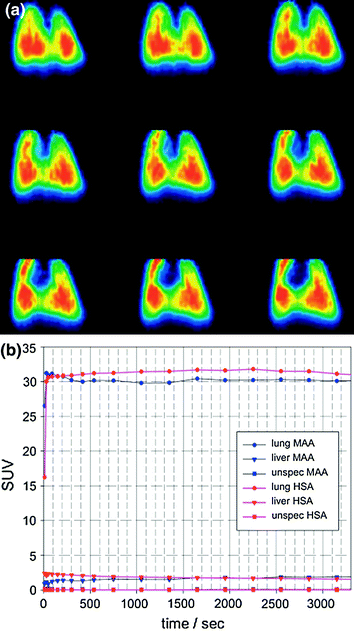



Fig. 2a, b
a PET images (coronal slices) of the lung 30–60 min after i.v. injection of Ga-68-labeled MAA using a dedicated microPET scanner Focus 120 (Sect. 2.3.1, Maus et al. 2011). b SUV curves after i.v. injection of Ga-68-labeled MAA and Ga-68-labeled HSA (Maus et al. 2011)


Fig. 2c, d
Lung (c) ventilation (top coronal slices, middle transaxial slices, bottom sagittal slices) and (d) perfusion (top coronal slices, middle transaxial slices, bottom sagittal slices) scan with an aerosol of Ga-68-labeled carbon particles (Galligas) and Ga-68-labeled MAA performed (n = 1) using a Gemini TF PET/CT scanner (Philips, Best, The Netherlands) showing complete incorporation in the lung of a Sprague–Dawley rat without any extrapulmonary activity being visualized
3.2.2 Clinical Study
In clinical use, PET lung ventilation and perfusion imaging using Ga-68 aerosol (Galligas) and Ga-68-labeled MAA was successful in all cases.
Ventilation imaging was homogeneous in two cases. In three cases, inhomogeneous accumulation with central deposition of radionuclide could be observed (Table 1).
Table 1
Clinical data and results of lung V/P imaging using PET/CT
Clinical symptoms | Ventilation | Perfusion | Pulmonary embolism (PE) | Additional information from low-dose CT |
|---|---|---|---|---|
77-year-old male with dizziness, decreased oxygen pressure, diabetic kidney disease, D-dimer at 5.66 μg/l | Homogeneous | Homogeneous | Excluded | Coronary atherosclerosis, calcification of the aortic valve, axial hiatal hernia, solitary gallstone, degenerative changes in the spine |
66-year-old female with heart failure NYHA class III, chronic obstructive pulmonary disease, extreme obesity, type II diabetes, previous artificial pacemaker implantation, previous acute kidney failure, previous PE and DVT, increasing dyspnea since 1 week, D-dimer at 1.24 μg/l | Inhomogeneous with central depositions and defects left lateral and in projection to horizontal fissure of right lung (Fig. 3a) | Inhomogeneous with defects left lateral and in projection to horizontal fissure of right lung (Fig. 3b) | Excluded, combined V/P defects left lateral with suspicion of pneumonia and in projection to horizontal fissure of right lung | Pleural effusion, ground-glass opacities particularly in left lower lobe, coronary atherosclerosis and severe aortic sclerosis, left ventricular dilatation, artificial pacemaker in the left upper chest |
70-year-old female with chronic asthma with acute dyspnea, D-dimer at 8.0 μg/l, creatinine at 2.02 mg/dL | Inhomogeneous with central radionuclide depositions (Fig. 3c) | Inhomogeneous, hypoperfusion in the right lower lobe dorsolateral (Fig. 3d) | Mismatch in terms of a PE | Pleural effusion, axillary lymph nodes increased but not enlarged, degenerative changes in the spine, calcification on the left lobe of the thyroid, enlarged left adrenal gland |
83-year-old male with acute dyspnea, two-level DVT, D-dimer at 4.4 μg/l, creatinine at 1.75 mg/dL, known prostate carcinoma | Homogeneous | Homogeneous | Excluded | Small pleural nodules, coronary atherosclerosis aortic sclerosis, multiple gallstones, suspect metastatic lesion in L1 vertebral body (Fig. 3f) |
80-year-old female with acute dyspnea, tachycardia, known thrombosis, renal insufficiency, D-dimer at 2.06 μg/l | Inhomogeneous with central and peripheral radionuclide depositions and defect in the left lower lobe | Homogeneous | Excluded, reverse mismatch in terms of pleural effusion with adjacent ventilation failure and suspicion of pneumonia | Severe pleural effusion with adjacent ventilation failure, dystelectasis and signs of lung infiltrate in the left lower lobe, severe aortic sclerosis and carotid artery stenosis, degenerative changes in the spine, calcified, right dorsal flexed uterus (Fig. 3i) |
The perfusion imaging was homogeneous in three cases. In one case, perfusion defects with corresponding ventilation defects were found (Fig. 3a, b).
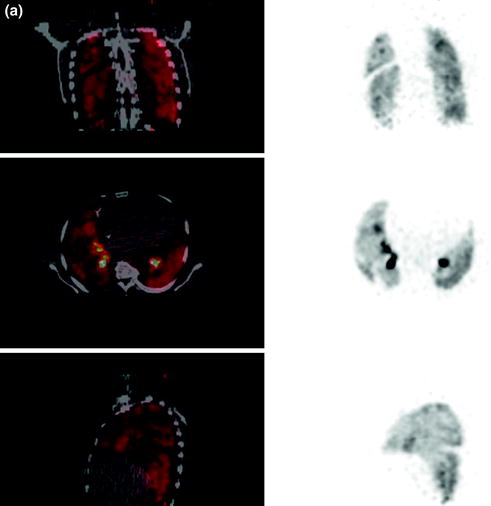
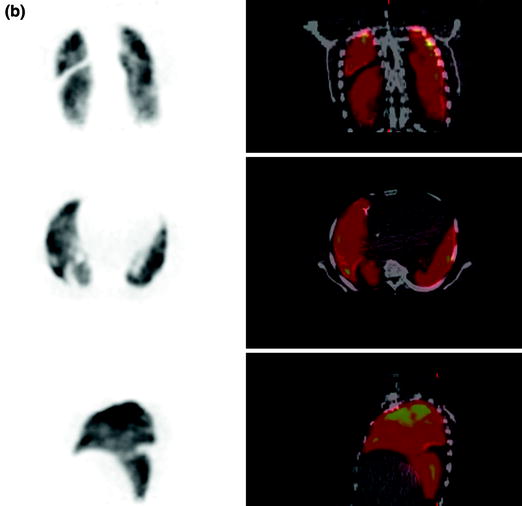
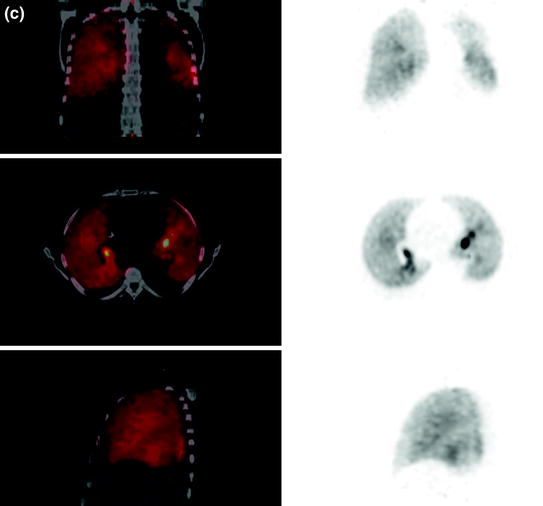
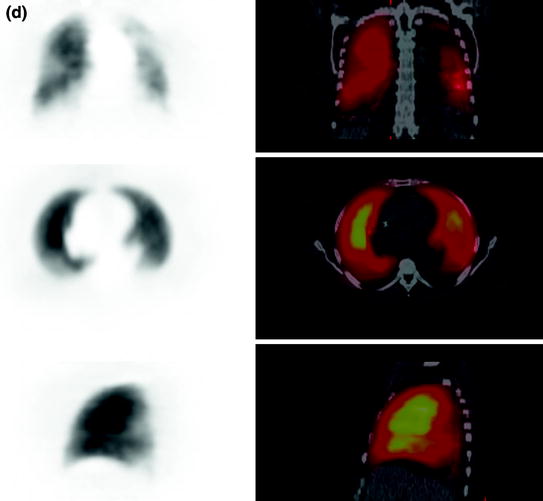
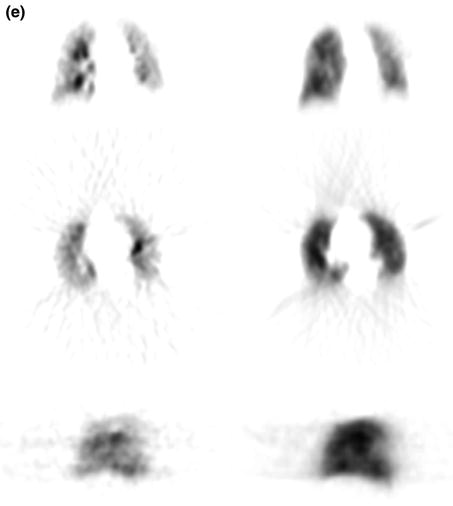
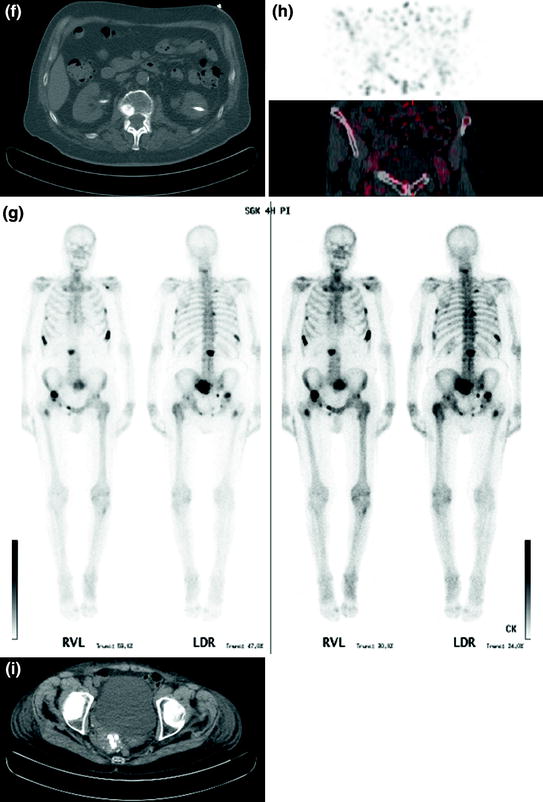


Fig. 3a, b
PET lung (a) ventilation and (b) perfusion imaging using Ga-68 aerosol (Galligas) and Ga-68-labeled MAA showing combined ventilation (top coronal slices, middle transaxial slices, bottom sagittal slices)/perfusion (top coronal slices, middle transaxial slices, bottom sagittal slices) defects left lateral and in projection to the horizontal fissure of right lung PET lung


Fig. 3c, d
c Ventilation (top coronal slices, middle transaxial slices, bottom sagittal slices) and (d) perfusion (top coronal slices, middle transaxial slices, bottom sagittal slices) imaging using Ga-68 aerosol (Galligas) and Ga-68-labeled MAA showing a hypoperfusion in the right lower lobe dorsolateral (especially segment 6) and preserved ventilation in the same region in terms of PE (mismatch defect)

Fig. 3e
Follow-up 3 days later (case 1). Left Ventilation imaging with Tc-99m aerosol (Technegas®); Right Perfusion imaging with Tc-99m-labeled MAA

Fig. 3f–i
f Low-dose CT with significant metastatic suspect lesions in L1 vertebral body (case 2). (g) Bone scintigraphy with multifocal metastatic bone disease (case 2). (h) PET scan of lower abdomen without visible activity in the urinary bladder (case 3). (i) Low-dose CT showing a calcified, right dorsal flexed uterus (case 3)
Case 1.
70-year-old female with acute dyspnea, elevated D-dimer to 8.0 μg/l, and creatinine to 2.02 mg/dL showed an inhomogeneous nuclide accumulation with a hypoperfusion in the right lower lobe dorsolateral (especially segment 6) and preserved ventilation in the same region in terms of PE (mismatch defect) (Fig. 3c, d). A follow-up study was performed with Tc-99m aerosol (Technegas®) and Tc-99m-labeled MAA 3 days later (Fig. 3e).
In all other cases PE as reason for the clinical symptoms could be excluded. Due to the low-dose CT performed for AC there was additional diagnostic information, for example, pleural effusion, infiltrates, dystelectasis, and more (Table 1).
Case 2.
83-year-old male with acute dyspnea, known two-level deep vein thrombosis (DVT), elevated D-dimer to 4.4 μg/l, and subsequent suspicion of PE. CT angiography (CTA) was not applicable in regard of creatinine elevated to 1.75 mg/dL. The V/P imaging showed homogeneous accumulation in both scans without any signs for PE. Nevertheless, low-dose CT showed a significant metastatic suspect lesion in L1 vertebral body (Fig. 3f) especially in the knowledge of an operated prostate carcinoma 2 years ago. Additional bone scintigraphy revealed multifocal metastatic bone disease (Fig. 3g).
Case 3.
80-year-old female with acute dyspnea, known thrombosis, renal insufficiency, and elevated D-dimer to 2.06 μg/l. PE as reason for the clinical symptoms could be excluded. The V/P imagings showed a reversed mismatch according to morphological signs of pneumonia. In this patient a third bed position was performed with the lower abdomen. No activity in the urinary bladder could be observed (Fig. 3h). Concomitant low-dose CT revealed a calcified, right dorsal flexed uterus with suspicion of uterine myomatosis (Fig. 3i).
3.3 Dosimetry
Dosimetry calculations were performed using OLINDA (version 1.1, Vanderbilt University, 2007). Residence times were calculated assuming purely physical disintegration of activity in the lung. Administered activity was recovered almost fully in the “whole-body” region of interest (ROI) of the PET images (in two or three bed positions) except for in one patient, in whom activity in the liver was detectable but not completely in the visual field.
On average, 7.5 MBq Ga-68 aerosol (Galligas) was inhaled (3.4–12.6 MBq), determined from the “whole-body” ROI (two or three bed positions). Previously, validation with a Ge-68/Ga-68 homogeneous cylinder phantom (27 MBq) was obtained.
The mean application activity for perfusion imaging was 70.4 MBq (42–105 MBq) as determined by decay-corrected measurement of the syringe before and after injection. The remaining activities were adjusted to the time difference between ventilation and perfusion imaging (19.6–23.7 min) and were included in the dosimetry calculations for perfusion imaging. This activity correction was 6 MBq on average.
Ventilation studies with Ga-68 aerosol (Galligas) showed a radiation exposure for the lung of 0.85 mSv/MBq and an effective dose of 0.11 mSv/MBq. According to the mean administered activity of 7.5 MBq, a lung dose of 6.4 mSv and an effective dose of 0.8 mSv could be calculated, respectively. In regard to image quality, 3.4 MBq inhaled Ga-68 aerosol (Galligas) was sufficient for ventilation imaging (Fig. 4a).
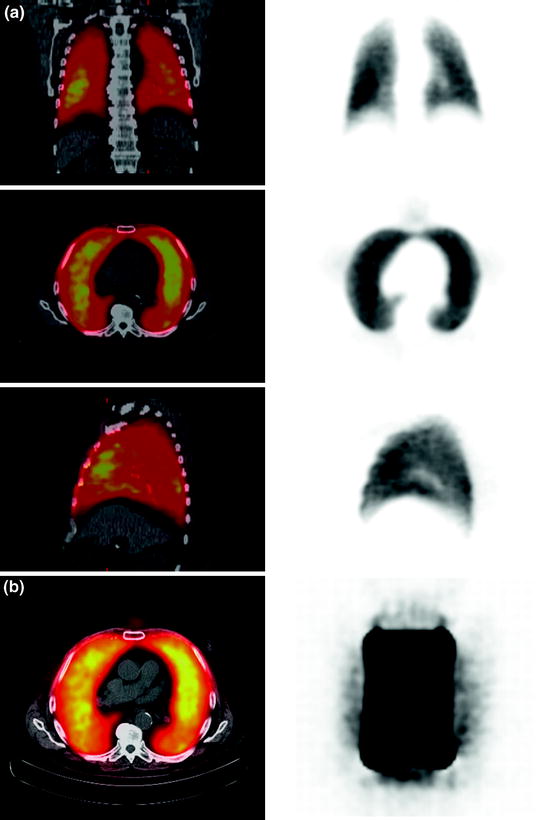

Fig. 4
a Ventilation imaging (top coronal slices, middle transaxial slices, bottom sagittal slices) performed with 3.4 MBq inhaled Ga-68 aerosol (Galligas). b Left “Extrapulmonary activity” could be visualized (ventilation imaging, transaxial slices; see a for coronal and sagittal slices) and verified in dosimetry calculations in the rib skeleton. Right Phantom measurements with lung mass density equivalent material (−850 HU, healthy lung: −910 to −700 HU) in a closed hollow cylinder showing inhomogeneous Ga-68 tracer accumulation. The hollow cylinder was in a torso phantom filled with (Ga-68-free) water. The quantification showed that 15% of the total applied activity dose was measurable (but “not existing”) in the water
The lung perfusion scans provided a lung dose of 0.71 mSv/MBq (0.09 mSv/MBq effective dose). With the mean administered activity of 70.4 MBq, a lung dose of 49.4 mSv and an effective dose of 6.6 mSv were calculated.
“Extrapulmonary activity” could be visualized and verified in dosimetry calculations in the rib skeleton (Fig. 4) and in one case in the liver but not completely in the visual field for further quantification. Further phantom measurements showed that quantification of Ga-68 could be difficult. Of the administered activity in a closed hollow cylinder with lung mass density equivalent material (Fig. 4) 15% was recovered outside in activity-free water (inside of the torso phantom).
Low-dose CT (120 kV, 0.5 s/rotation, 5 m slices, max. 60 mAs, DLP 73.9–132.9 mGy·cm, field of view 600 mm) were obtained for AC with additional radiation exposure of 1.5 mSv on average.
4 Discussion
4.1 68Ga-Labeled Radiotracer for Lung Ventilation and Perfusion Imaging
4.1.1 Ga-68 Aerosol (Galligas)
The feasibility of producing radioactive-labeled carbon aerosol with other radionuclides (F-18, K-42, Cu-64, Ga-68) using a Technegas® generator was already investigated by Nozaki et al. in 1995. The latest preparation and assessment of F-18 fluorogas for lung scintigraphy was presented by Henriksen et al. (2011). Three healthy male volunteers underwent PET/CT scan after inhalation of >37 MBq fluorogas (185 MBq loaded into the crucible). Homogeneous distribution of activity over the entire lung parenchyma was described. These findings correlate with the data of Nozaki et al. (1995), who demonstrated a good distribution of F-18-labeled carbon particles in the lung periphery, but also rapid elimination from the lungs with half-life of 10 min. F-18 had labeling yield of 20–25%. In contrast, Ga-68 and Tc-99m showed similar labeling yields of 30–45% and a similar washout fraction in cold water of 5–15% and <15%, respectively. The combination of graphite and an argon atmosphere was used to vaporize generator-eluted [Ga-68]Ga-chloride for generating Ga-68-labeled particles. The assumption was that charged Ga-68 binds by adsorption to the carrier particles (carbon particles) in the aerosol (Nozaki et al. 1995). The physical and chemical nature of Technegas® were described in detail by Senden et al. (1997). The generated particles consist of hexagonal platelets of metallic Tc-99m, which are tightly enclosed by a thin layer of carbon. The average size of the particles is about 30–60 nm with approximately 80% of particles smaller than 100 nm. The ratio of thickness to diameter is approximately 1:10. Similar findings are expected for Ga-68-labeled carbon particles (Galligas).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree