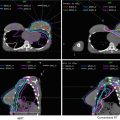
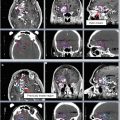
Volume
Description
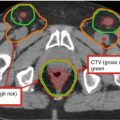
Gross tumor volume (GTV)
Pre-chemotherapy/surgery GTV (GTVp)
Contains gross tumor volume as identified on diagnostic imaging prior to chemotherapy and/or surgery
Post-chemotherapy/surgery GTV (GTVr)
Contains gross tumor volume as identified on diagnostic and/or planning imaging after management with chemotherapy and/or surgery
No prior treatment GTV (GTV)
Contains gross tumor volume identified on diagnostic and/or planning imaging in the absence of prior chemotherapy or surgery (e.g., in the case of primary or salvage RT)
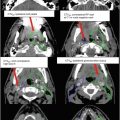
Clinical target volume (CTV)
Contains entire post-chemotherapy/surgery or “no prior treatment” GTV
Generally contains pre-chemotherapy/surgery GTV (unless volume is unable to be safely treating in its entirety)
Excludes extent of pre-chemotherapy/surgery GTV that displaced normal, uninvolved tissue (bone, organs, muscles, etc.) prior to chemotherapy or surgery
Includes consideration of the following:
Image accuracy and quality. CTV should be increased in size to account for uncertainties such as suboptimal fusion of pre- and post-chemotherapy/surgery images to planning images or in the absence of pre-chemotherapy/surgery images
Changes in volume since the time of imaging
Pattern of disease spread
Potential subclinical involvement. Consider including nodes of unknown status near the site of disease, particularly if the questionable nodes belong to a nodal chain or group already partially encompassed by the CTV
Adjacent organ dose constraints
Can contain separate nodal volumes if ≤5 cm apart. Lesions >5 cm apart are treated with separate CTVs
Internal target volume (ITV)
Contains the CTV plus an internal margin that accounts for variation in CTV shape, size, and position (e.g., target movement with respiration)
Most relevant for targets within the chest and abdomen; may be unnecessary for other sites
Planning target volume (PTV)
Contains CTV or ITV plus a margin to account for setup uncertainties associated with patient position and beam alignment
Note that CTV to PTV expansions are patient- and institution-specific; any recommendations in this chapter or elsewhere must be adjusted to reflect factors unique to the patient and institution
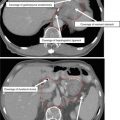
Organs at risk (OAR)
Includes uninvolved normal structures at risk of RT-related toxicity for which RT planning or dose may be altered
2 Anatomy and Patterns of Spread
Hodgkin lymphoma is a malignancy of the lymphatic system. Consequently, the pattern of spread generally follows that of the lymphatic system in a predictable manner. The most common sites of disease are the mediastinum and cervical/supraclavicular regions.
Although pretreatment imaging may occasionally show non-contiguity, subclinical disease is likely to be harbored in the lymphatic region lying between two regions shown to be involved on CT or PET/CT scan.
Splenic involvement almost always accompanies para-aortic involvement. Splenic disease is always present if there is bone marrow or liver involvement.
Bone marrow involvement occurs more commonly in patients with B symptoms and more advanced disease. PET/CT scan is sufficient for diagnosing bone marrow involvement, allowing patients to forgo bone marrow biopsies.
3 Diagnostic Workup Relevant for Target Volume Delineation
All patients should undergo a contrast-enhanced CT of the neck, chest, abdomen, and pelvis along with a PET/CT scan prior to starting any systemic therapy (including steroids). Nodes that are palpable on exam, enlarged on CT, or positive on FDG-PET are considered to be positive. Unusual or non-contiguous sites of disease should be biopsied to confirm involvement.
(i)
When possible, PET/CT scan and contrast CT scan should be performed in the treatment position to assist in fusion with the CT simulation scan following chemotherapy. This may require careful coordination with radiology and medical oncology and developing a special “lymphoma” imaging protocol to be used when a patient is undergoing staging for lymphoma or when lymphoma is high on the differential diagnosis list.
1.
Arms slightly akimbo or at the side allow for more reproducible setup and fusion with CT scan, when axillary nodes are involved. However, arms above the head may provide better access to the mediastinum if IMRT or rapid arc is contemplated.
2.
Neutral or slightly extended neck, which allows for potentially less mandible to be included in the treatment field. Hyperextended neck may drop the brain further into the field when the high cervical nodes are involved. Diagnostic scans are often done in a head-neutral or head-flexed position.
3.
Performing the initial staging scans with deep inspiration breath-hold technique may allow for further reduction in radiation dose to the heart and lung.
Chest X-ray still is the preferred way to characterize mediastinal bulk, due to its use in prior clinical trials. Masses greater than one-third of the maximum intrathoracic diameter or greater than 10 cm are considered to be unfavorable.
MRI and MRI/PET scans may also be used, especially in pediatric Hodgkin lymphoma. Although these modalities will reduce the radiation dose compared with a PET/CT scan, they may not fuse as well with CT simulation and may, in fact, lead to larger radiation targets, due to the uncertainties with the fusion. FDG-PET remains the most effective way to define initial sites of disease.
One should familiarize themselves with the Deauville criteria for evaluating treatment response in Hodgkin lymphoma (1, no uptake; 2, uptake < = mediastinum; 3, uptake > mediastinum but < = liver; 4, uptake > liver at any site; 5, uptake > liver and new sites of disease).
4 Simulation and Daily Localization
The vast majority of patients with Hodgkin lymphoma will undergo radiotherapy as consolidation following chemotherapy. Since target delineation is based on pre-chemotherapy sites of involvement and since disease is expected to regress following chemotherapy, improved image registration with the pre-chemotherapy staging scans will allow for reduced size in target volume (due to less margin for fusion uncertainty). However, repositioning the patient may be required to avoid the OARs, despite requiring more margin for fusion uncertainties. For example, if the pretreatment PET/CT scan is performed with the head flexed, maintaining the same position for treatment would lead to unnecessary irradiation of the salivary glands and oral cavity in the presence of high cervical nodal disease. Planning treatment with the neck in a different position than the pretreatment PET/CT will require increasing the size of the CTV because of the uncertainty in image registration.
When the supraclavicular or cervical region is involved, a face mask is used to help stabilize the patient in a reproducible manner and keep the chin from falling into the treatment field.
(i)
When a face mask is used, it is advisable to avoid putting arms above the head, as this can cause significant pain and discomfort for some patients during their treatment.
When axillary nodes require treatment, it is important to try and reproduce the initial staging scan patient setup. Although axillary nodes that were initially involved might be quite small, the axilla is a large nodal station, and the nodes can move considerably depending on patient arm position. Since these nodes may be difficult to identify at the time of simulation, larger margins will need to be used when discrepancies in arm setup are present between the staging scan and CT simulation. In this situation, it may be preferable to have the arms at the patient’s side with a Vac-Lok bag and arm pulls to prevent the patient’s arms from drifting during treatment.
CT simulation should be performed with at a minimum 3 mm slices, starting at least 5 cm above the most superior level of disease from the staging scans and end at least 5 cm below the most inferior level of disease from the staging scans. Make sure to include adjacent OAR in their entirety to ensure an accurate DVH (i.e., include the entire lungs, even if the entire mediastinum is not included).
IV contrast can help differentiate between residual Hodgkin lymphoma and the OARs and major vessels. It may also be helpful for contouring cardiac structures, including coronary vessels and valves.
Wire scars from biopsies to define prior lymph node involvement, especially when the staging scans were done after the patient had an excisional biopsy.
When the mediastinum, lung, para-aortics, and/or spleen are to be irradiated, one should consider respiratory motion management strategies. These may include:
(i)
4D CT simulation to assess respiratory motion and either develop the GTV and CTV based on all 10 phases of the respiratory cycle or consider adding a uniform superior-inferior margin to the GTV and CTV drawn on the average scan to account for motion throughout the breathing cycle
(ii)
Deep inspiration breath-hold technique
(iii)
Abdominal compression (may increase lung dose when mediastinum or lung undergoing treatment, due to smaller lung volumes)
Deep inspiration breath hold might be considered for patients with mediastinal involvement to help pull the heart away from the disease involvement and to increase lung volume, which may help reduce overall dose to the heart and lungs. This must be done with care, and it is important to have the staging CT scan performed with a breath-hold technique, to reduce uncertainties in image registration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree