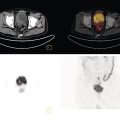
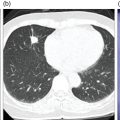
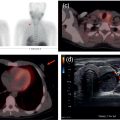
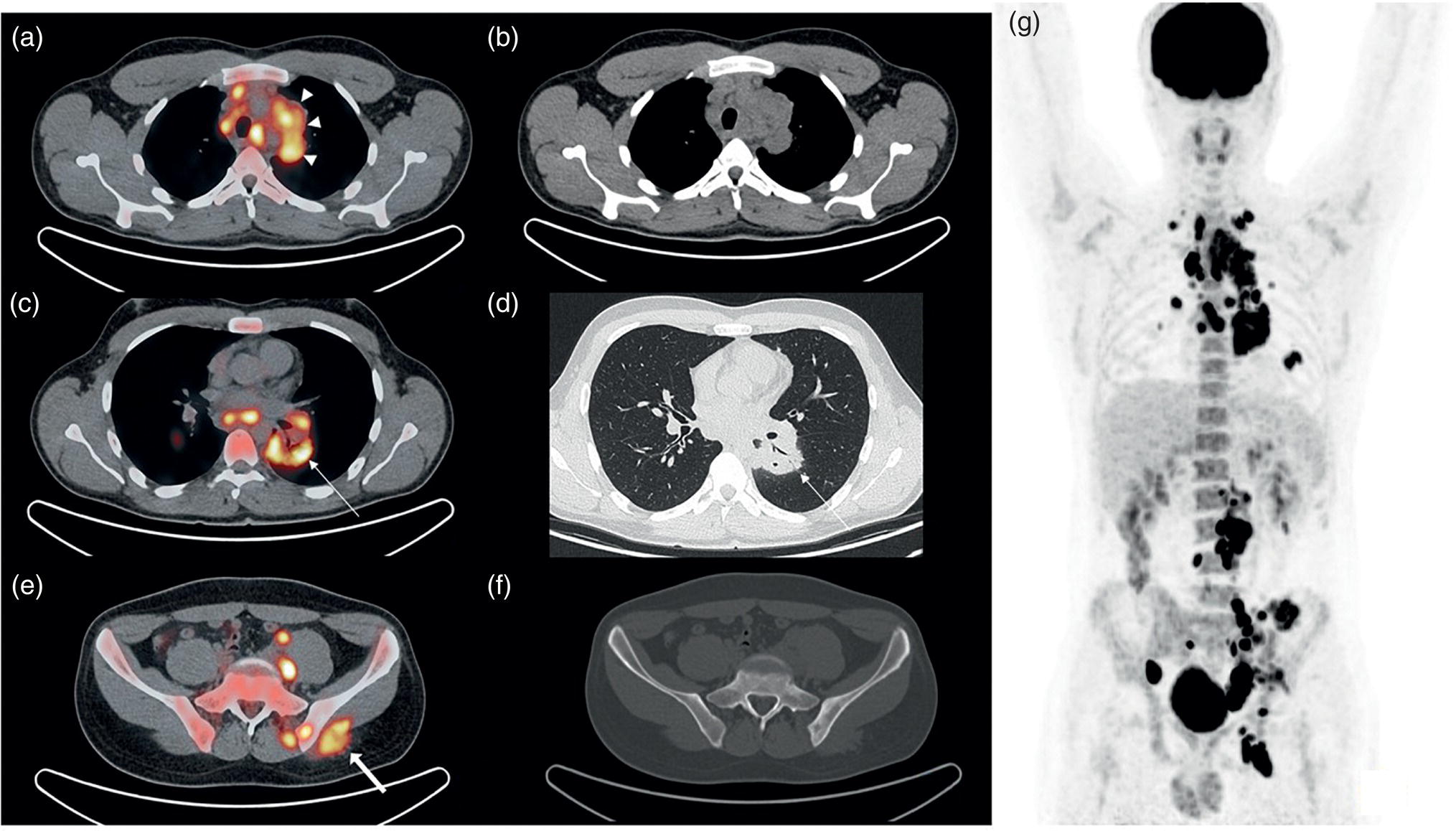
Pavel Gelezhe1,2, Sergey Morozov6, Anton Kondakov3,4, and Mikhail Beregov5 1 Research and Practical Clinical Center for Diagnostics and Telemedicine Technologies of the Moscow Health Care Department, Moscow, Russia 2 European Medical Center, Radiology Department, Moscow, Russia 3 Central Clinical Hospital of the Russian Academy of Sciences, Nuclear Medicine Department, Moscow, Russia 4 Pirogov Russian National Research Medical University, Department of Radiology and Radiation Therapy, Moscow, Russia 5 Federal Center for Cerebrovascular Pathology and Stroke, Department of Radiology and Functional Diagnostics, Moscow, Russia 6 Chief innovation officer, Osimis S.A., Belgium Lymphoma presents as pathologic growth of lymphoid cells or their precursor forms. The 2016 WHO Classification divides mature lymphoid, histiocytic, and dendritic neoplasms into five major subtypes: (i) mature B‐cell neoplasms, (ii) mature T‐cell and NK‐cell neoplasms, (iii) Hodgkin lymphoma (HL), (iv) posttransplant lymphoproliferative disorders (PTLD), and (v) histiocytic and dendritic cell neoplasms [1]. These tumors were first described by Thomas Hodgkin in 1832, and one of the lymphoma subtypes was subsequently named after him [2]. HL accounts for 10–30% of all lymphoma cases [3, 4] and is divided on the basis of phenotypic features into two categories: classic Hodgkin lymphoma (cHL), which represents 95% of all HL cases, and nodular lymphocyte predominant Hodgkin lymphoma (NLPHL), characterized by indolent progression and total replacement of nodal architecture. Reed–Sternberg cells and Hodgkin cells are histologic hallmarks of HL [3]. All other cases of lymphomas were previously recognized as non‐Hodgkin lymphoma (NHL) and this term is still in use in current publications, textbooks, and the 8th edition of the TNM classification [5, 6]. NHL is the most common hematologic malignancy in the world. To date the WHO recognizes more than 70 unique subtypes of these tumors divided by their histologic and immunophenotypic features [1]. Epidemiological and genetic studies suggest that lymphoma has multiple etiologies, with certain subtypes linked with Epstein–Barr virus (EBV), human T‐lymphotropic virus (HTLV), human immunodeficiency virus (HIV), and some other causes, such as immunosuppressive medications [7, 8]. Lymphoma has bimodal age distribution (first peak at 15–20 years, second one later in life, around 55–65 years) and slight male predominance [9]. For all lymphomas common symptoms are painless lymphadenopathy, loss of appetite, anorexia, fatigue, itching, dry cough, pain or obstruction in case of local pressure in a mass‐like type of growth or other nonspecific symptoms. There are also three systemic symptoms: fever (oral temperature more than 38.0 °C), night drenching sweats, and unexplained weight loss (>10% from baseline, 6 months before staging), which are included into a subgroup of B‐symptoms [3]. This symptom subgroup is used in staging and registered in about 40% of lymphoma cases [9]. According to current 8th edition of the TNM staging system for both HL and NHL, stage estimation is based on the Cotswolds‐modified Ann Arbor staging system. There are four stages indicating the extent of involved lymph nodes levels as well as the presence of malignant cells in other organs. If B‐symptoms are evident, a “B” suffix is used for stage determination. Their absence is denoted as the “A” suffix. TNM 8th edition recommends against indicating of B‐symptoms in staging of NHL [5]. Suffix “E” is added if extralymphatic site of disease is evident. It should be noted that one lobe involvement and perihilar extensions in lungs are described as localized extralymphatic disease. On the contrary, liver involvement is always described as diffuse extralymphatic disease. Thymus, spleen (designated by an “S” suffix in the stage), and appendix are classified as lymphatic regions [5]. Disease is called “bulky” if it has nodal mass greater than 10 cm maximum dimension for HL and diffuse large cell lymphoma, and 6 cm for follicular lymphoma (FL). Bulky mediastinal disease is most common in young women with nodular sclerosis cHL [5]. Stage I (or IE) indicates one site of disease. Stage II is used for two, three, or more involved nodal regions on the same side of the diaphragm or localized involvement of extralymphatic sites (stage IIE) with or without regional lymph node involvement. The term “limited stage” embodies all of above entities [5]. Stage III indicates that lymphatic regions are involved on both sides of the diaphragm (IIIS if the spleen is involved). Stage IV stands for disseminated disease of one or more extralymphatic organs with or without lymph node involvement or noncontiguous extralymphatic organ involvement with involvement of lymph node regions on the same or both sides of the diaphragm [5]. HL is a highly curable disease with almost 90% five‐year survival rate in developed countries [10, 11]. NHL has a 69% 5‐year survival rate, which varies depending on lymphoma histologic type [10]. A common treatment in lymphoma is multidrug chemotherapy, sometimes accompanied by radiation therapy. In nonbulky limited stage HL first‐line therapy is a combination of doxorubicin (trade name Adriamycin), bleomycin, vinblastine, dacarbazine (ABVD) given in three to four cycles. In advanced stages of the disease it is recommended to use more cycles of ABVD or other types of chemotherapy such as an escalated BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) followed by radiotherapy [12]. NLPHL suggests observation after surgical resection of the involved lymph node, radiotherapy, rituximab and two to four cycles of chemotherapy at early stages and R‐CHOP chemotherapy in the advanced stages [12]. Programmed cell death‐ligand 1 (PD‐L1) and PD‐L2 are overexpressed on lymphoma cells in classic HL, which may have the potential benefit of checkpoint inhibitor administration such as pembrolizumab and nivolumab, but the evidence is insufficient to recommend these treatments except in patients who have recurrent disease after salvage chemotherapy followed by autologous stem cell transplantation [12, 13]. Brentuximab vedotin is a drug‐antibody conjugate used in refractory HL with a high response rate of 75% [12, 14]. Radiotherapy in HL usually includes involved‐field radiotherapy (IFRT, 20–30 Gy) to augment chemotherapy. Radiation oncologists should use smaller doses and smaller fields of irradiation to eliminate late side effects, predominantly in heart and lungs, especially in a young population of HL patients [12]. NHL has many treatment protocols depending on its clinical and pathological features. One common agent for B‐cell lymphoma is rituximab, a chimeric monoclonal antibody to CD20 antigen. It is used alone or in combination with other chemotherapy regimens such as BR (bendamustine and rituximab), R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), R‐CVP (rituximab, cyclophosphamide, vincristine, and prednisone), and some others [15]. Radiotherapy has wider use in NHL but the doses are generally higher than those for HL (30–40 Gy) [16]. Positron emission tomography‐computed tomography (PET/CT) with 18F‐fluorodeoxyglucose (FDG) is generally recognized as the routine imaging workup for lymphoma patients in staging, interim, and end‐of‐treatment response assessment. In some cases, tumor cells are not FDG‐avid, so CT with contrast enhancement is preferred for response assessment [6, 12, 17]. Glucose and 18F‐FDG are taken up by metabolically active cells by means of glucose transporters (GLUT) and then are phosphorylated by hexokinase. Due to structural differences FDG cannot be rearranged by glucose phosphate isomerase and is trapped in the cytoplasm. Cancerous cells such as lymphoma cells have a high rate of glucose utilization compared with normal cells because of inefficient methods of glucose utilization: anaerobic glycolysis instead of oxidative phosphorylation even in normoxic conditions (the so‐called Warburg effect) [18, 19]. Intense uptake of FDG in PET allows not only direct cancer visualization but also semiquantitative measurement of its metabolic activity and provides superior sensitivity compared to CT and magnetic resonance imaging (MRI). The sensitivity of PET/CT with FDG in FDG‐avid neoplasms indicates sites of disease that do not meet size criteria or cannot be found with CT alone, like bone [17]. Other nuclear medicine procedures can be used in lymphoma. High uptake of gallium‐67 (67Ga)‐citrate in lymph nodes in different types of lymphoma was first described by C.I. Edwards and R.L. Hayes in 1969 [20]. 67Ga‐citrate imaging was used as an adjunction to diagnostics of lymphoma for a few decades after that due to its high sensitivity (almost 90% above diaphragm) and possibility of viable cancer tissue visualization at restaging and therapy efficiency assessment [21]. The exact mechanism of 67Ga‐citrate localization in tumors is not known, but high capillary permeability for transferrin–67Ga complex, its binding to tumor cell receptors, and slower washout from the cytoplasm of malignant cells possibly play significant roles [22]. Limitations of 67Ga‐citrate imaging include low spatial resolution of conventional whole‐body scan and poor sensitivity at the subdiaphragmatic level due to physiologic uptake of the radiopharmaceutical in liver and spleen. Single‐photon emission CT may be useful to overcome this limitation but its spatial resolution is still lower than that in PET. Another drawback of this method is the long time between injection and scan: it takes 2–3 days for 67Ga‐citrate to be distributed in the body [21]. Bone involvement in lymphoma may be demonstrated by bone scan with technetium‐99m (99mTc)‐methylene diphosphonate (MDP) or other bone‐seeking agents. This is not a recommended method nowadays, but lymphoma may be detected occasionally as an incidental finding [23]. In general, bone involvement on bone scan shows higher uptake compared to contralateral or adjacent regions and depends on the level of osteoblastic activity in the lesion. In pure osteolytic lesions bone scan has nearly no sensitivity except in cases of pathological fractures [24, 25]. The patient is advised to avoid strenuous physical exercise for 24 hours before the procedure. The patient should fast for at least 5 hours before the procedure and should follow a low‐carbohydrate diet with adequate hydration on the day before PET/CT. Patients with diabetes should discontinue insulin on the day of the procedure for at least 4 hours before FDG injection. The serum glucose level should be lower than 11 mmol/l (about 200 mg/dl) for a good‐quality diagnostic study [19]. The usual scan from base of skull to mid‐thigh is enough for correct staging and response assessment. In certain cases, the length of the scan may be extended to cover skull and legs, especially in cases of possible involvement. Additional contrast‐enhanced CT (CECT) may be used for more accurate delineation of involved sites and for diagnosis of thrombosis or occlusion in compression of vessels by enlarged lymph nodes, radiation therapy planning, and some other conditions. Many patients have recent CECT made before PET/CT, so there is no need to perform it along with PET/CT [17, 19]. If not, CECT may be performed along with PET/CT, but the risks of additional radiation exposure and expected benefits should be compared [17]. CECT may be used for attenuation correction in PET [26]. Despite the possibility of visualizing brain structures and glucose uptake in them, primary central nervous system (CNS) lymphoma should not be evaluated with 18F‐FDG‐PET/CT because of high background uptake. Contrast‐enhanced MRI should be used instead [27]. Current guidelines (Lugano classification) indicate that PET/CT with FDG should not be used in certain histologic types of lymphoma, such as chronic lymphocytic leukemia/small lymphocytic lymphoma, lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia, mycosis fungoides, and marginal zone NHLs, unless there is a suspicion of aggressive transformation [6]. In other cases the radiologist should perform a visual analysis to find all sites of the disease and exclude those which are linked to physiologic or nonlymphomatous uptake. As described above, staging of lymphoma is based on the Ann Arbor staging system, so radiologists should follow this system in their reports. Some clinical sites use a five‐point scale (Deauville score) to measure the intensity of FDG uptake in nodes, although this has not been validated for the initial staging purposes [6]. SUV measurements (SUVmax or SUVpeak, corrected for the patient’s weight or lean body mass) are not obligatory at initial staging but are sometimes useful for assessing lymphoma aggressiveness, especially in cases of presumed indolent lymphoma or to indicate the preferred biopsy site [28, 29]. If the tumor turns out to be not FDG‐avid, staging should be performed on the basis of CECT findings with reporting of the longest and shortest diameters of up to six nodal or extranodal measurable lesions (with the longest diameter of at least 1.5 cm for lymph nodes and 1.0 cm for extranodal lesions) [6]. An illustration of the initial staging of histologically confirmed Hodgkin lymphoma is shown in Figure 28.1. Current guidelines advise that PET/CT should be performed during chemotherapy for both HL and NHL with FDG‐avid histology. This type of study is called interim PET/CT (iPET/CT). Glucose metabolism is interrupted in cancer cells during chemotherapy infusion and few days after it, so iPET/CT should be performed 7–10 days after the last chemotherapy infusion of the second or third cycle to avoid false‐negative results [6, 19]. Figure 28.1 Male patient, 23 years old. Initial staging of histologically confirmed Hodgkin lymphoma. Fusion images (a, c, e) and corresponding CT images (b, d, f) are presented. (g) The maximum intensity projection image. FDG‐PET/CT reveals conglomerates of multiple enlarged lymph nodes on both sides of the diaphragm, including those in the upper mediastinum (a, arrowheads). Extranodal lesions are present: a mass‐like consolidation in the perihilar region of the left lung (c and d, thin arrow), infiltrative lesion of the left gluteus maximus muscle (e, thick arrow). For example, ABVD chemotherapy is performed as 28‐day cycles with infusions made on the first and 15th days, which means that optimal timing for iPET/CT is 22–25 day of the cycle [17]. Lugano classification recommends use of a five‐point scale, known as the Deauville score, for therapy response assessment. The Deauville score was developed in 2009 and since then has been widely applied in clinical trials and routine practice as a simple visual scale [6, 30]. Mediastinal blood pool and intact liver tissue are the reference regions which are used to visually evaluate lesion uptake. Any pathological radioactivity in a residual lesion is compared with these two reference regions and graded according to Table 28.1 [6]. To measure the reference SUVs one should place the region of interest (ROI) in the arch of the aorta or above the aortic root far from vessel walls or calcified plaques. The liver ROI should not be close to the liver margin and should not include any noise pixels with unusually high uptake. For the best quantitative assessment ROIs might be placed on several axial slices. When the prescribed course of treatment is finished the patient should undergo an end‐of‐treatment response assessment, which seems to be more accurate than CT alone. The end‐of‐treatment PET/CT (ePET/CT) should be performed at least 3 weeks after the end of chemotherapy but preferably 6–8 weeks after the end of chemotherapy. A minimal interval of 3 months is required after the completion of radiotherapy [17]. The Lugano classification also proposes use of the Deauville scale (Table 28.1) to evaluate the end‐of‐treatment response. Based on it, the following categories can be distinguished: Table 28.1 Deauville five‐point score and its interpretation. ePET/CT, end‐of‐treatment PET/CT; iPET/CT, interim PET/CT; MBP, mediastinal blood pool. Pseudoprogression represents the phenomenon of uptake increase or lesion enlargement due to immune cells infiltrating the tumor tissue with subsequent delayed tumor regression. It often occurs during immunotherapy, which stimulates immune recognition of tumors, as in the case with checkpoint inhibitors [31]. The first PET/CT study should be performed 12 weeks after immunotherapy initiation. In 2016 the Lugano classification was adopted for the evaluation of immunotherapy response. These refined criteria were named the Lymphoma Response to Immunomodulatory Therapy Criteria (LYRIC). They are provisional and not recommended for routine clinical practice [32]. The main difference is that a new category, indeterminate response (IR), was introduced. It comprises three following patterns: In the case of correspondence of presented features to more than one IR pattern, priority is given to IR(1) or IR(2) over IR(3). If any IR category is assigned, a biopsy or repeating PET/CT after another 12 weeks should be recommended to distinguish between true progressive disease and pseudoprogression. Figures 28.2–28.4 illlustrate the initial staging of histologically confirmed Hodgkin lymphoma, diffuse large B‐cell lymphoma, and classic Hodgkin lymphoma with thymic involvement.
28
Lymphoma and Myeloma Correlative Imaging
Lymphoma
Molecular Imaging Procedures in Lymphoma
Technical Considerations for the PET/CT Procedure (Patient Preparation, Scan Protocols)
Lymphoma Staging
Example
Response Assessment
Interim Response
End‐of‐Treatment Response (Timing of Study after RT and Chemotherapy, Complete and Partial Response, Residual Tissue)
Score
Definition
Response at iPET/CT
Response at ePET/CT
1
No uptake above the background
Complete metabolic response (with no bone marrow lesions)
Complete metabolic response (with no bone marrow lesions)
2
Uptake in residual tissue (initial lesion) is lower than MBP uptake
Complete metabolic response (with no bone marrow lesions)
Complete metabolic response (with no bone marrow lesions)
3
Uptake in residual tissue is less than liver uptake but higher than MBP uptake
Complete metabolic response (with no bone marrow lesions), except de‐escalation clinical trials
Complete metabolic response (with no bone marrow lesions)
4
Uptake in residual tissue is higher than liver uptake
Partial response (if uptake is decreased compared to baseline and there is no structural progression) or treatment failure in other cases
Residual disease if uptake is decreased or treatment failure if uptake is increased and there is no structural progression
5
Uptake in residual tissue is markedly higher than in liver (5a) or
There are new lesions (5b)
Partial response (if uptake is decreased and there is no structural progression) or treatment failure in other cases
Residual disease if uptake is decreased or treatment failure if uptake is increased and/or new lesions appear
Immunotherapy Response
Examples
Pitfalls
False‐positive
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree