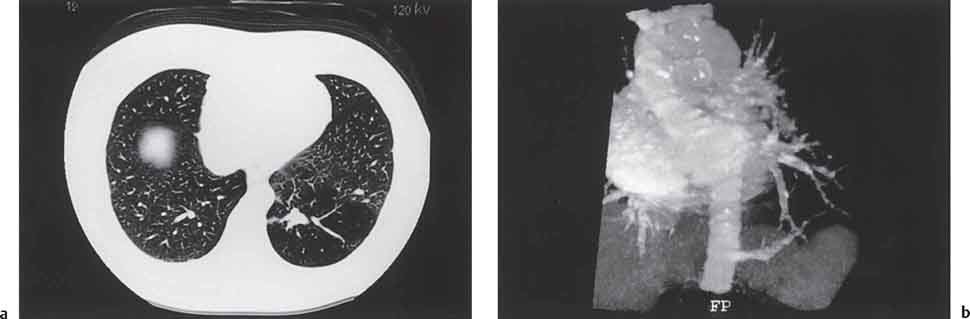
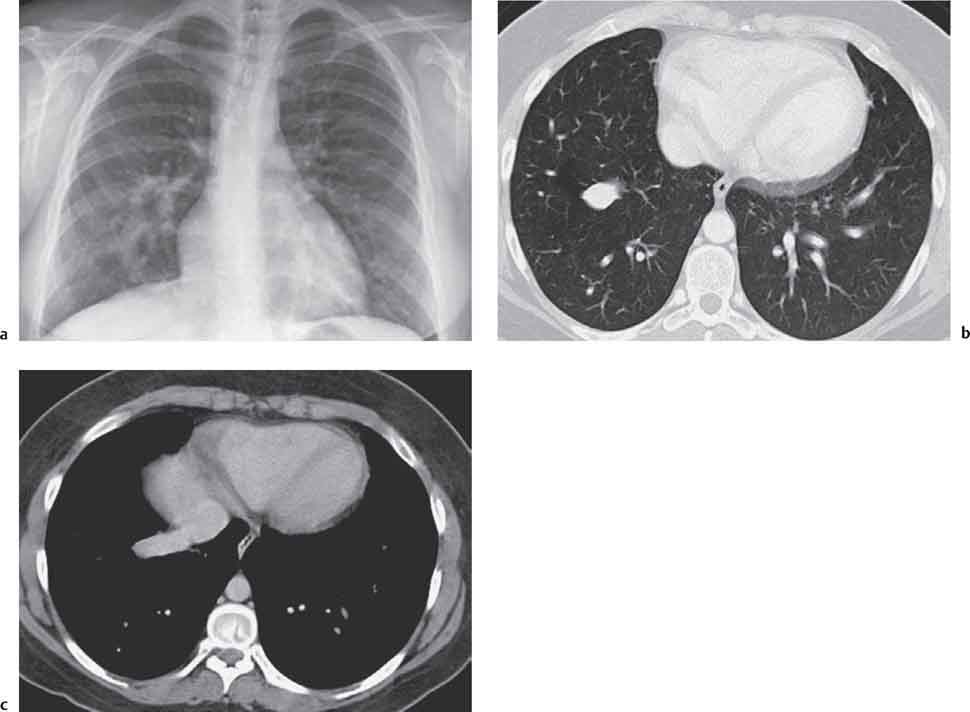
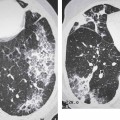
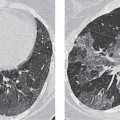
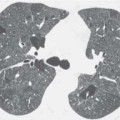
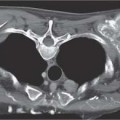
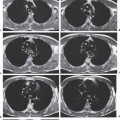
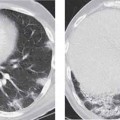
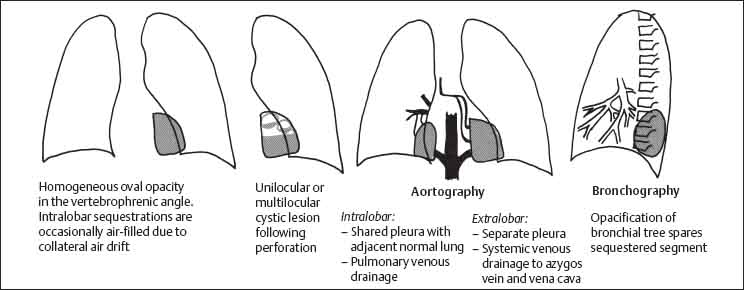
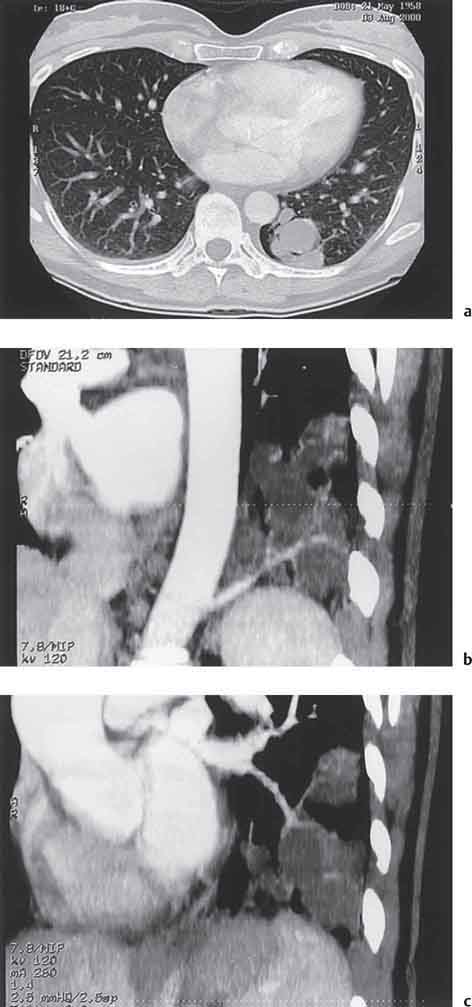
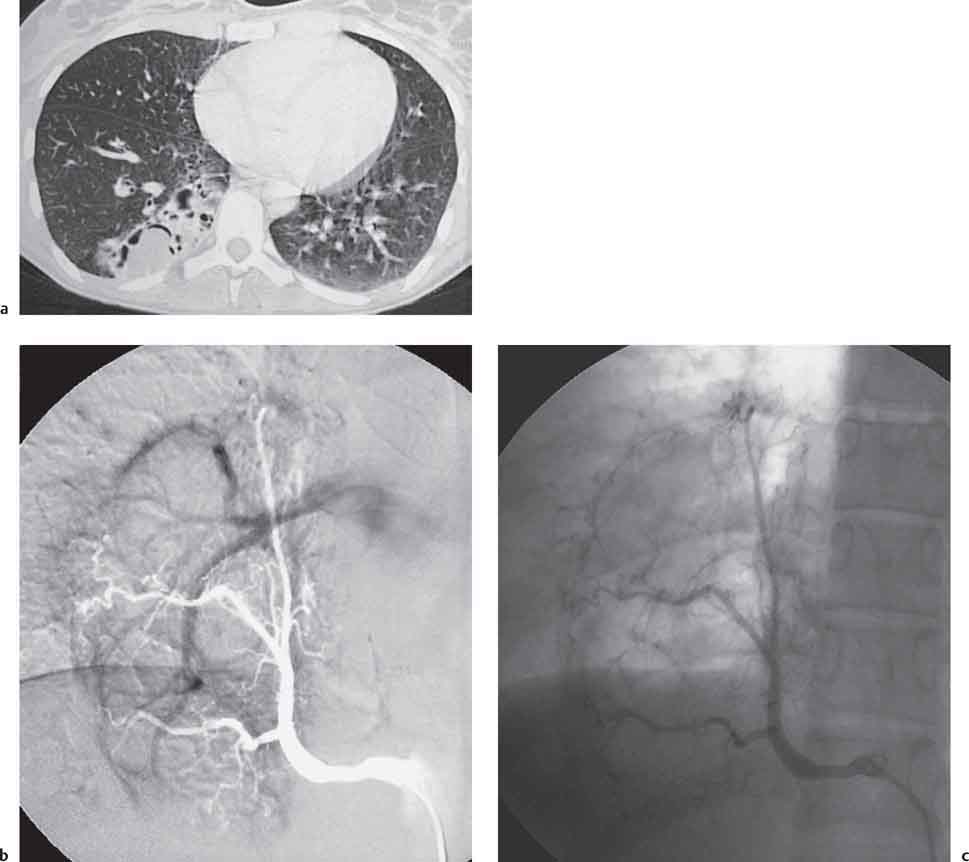
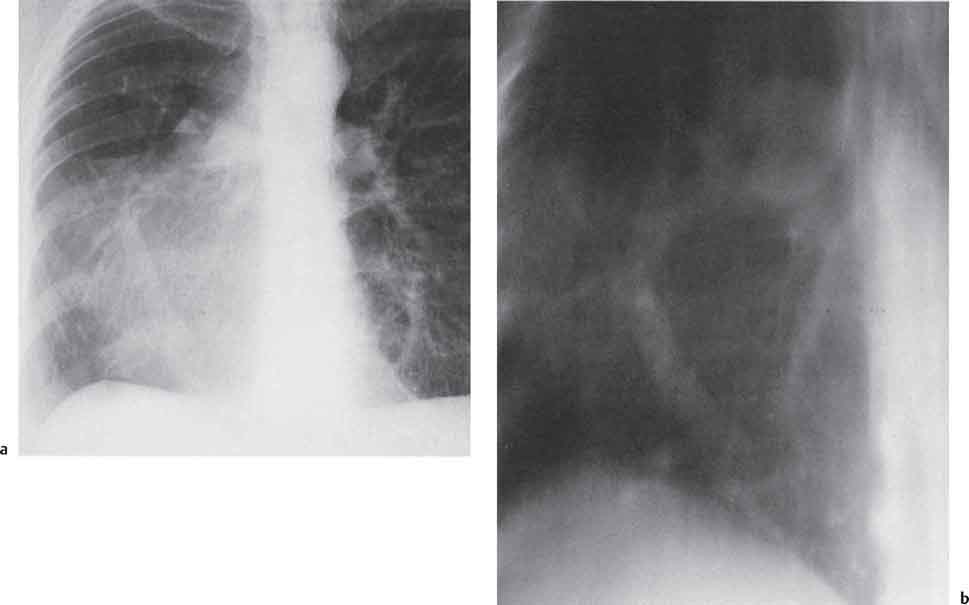
2 Malformations Pulmonary malformations are rare and frequently associated with extrapulmonary anomalies. Three main categories are recognized: Bronchopulmonary sequestrations represent areas of nonfunctioning lung tissue that do not communicate with the tracheobronchial tree and have a systemic arterial blood supply. Embryologically, pulmonary sequestrations are due to anomalous ventral budding from the foregut or tracheobronchial tree. Occasionally, they have a fistulous connection to the esophagus or stomach. Sequestrations are classified as extra- or intralobar in type. Extralobar malformations constitute 25% of sequestrations. They are discrete accessory lobes of nonaerated lung invested within their own pleural covering (Rosado de Christensen et al. 1993). They are located most commonly between the lower lobe and the diaphragm. They also may have an intradiaphragmatic, mediastinal, or intra-abdominal position. Extralobar sequestrations have a systemic arterial supply from branches of the aorta and a systemic venous drainage to the azygos veins or inferior vena cava. Associated anomalies including diaphragmatic hernia and bronchogenic cyst may be present in up to 60% of cases. Fig. 2.1 Pulmonary sequestrations. Intralobar sequestrations usually have a systemic arterial supply and pulmonary venous drainage. Sixty percent of intralobar sequestrations are left-sided and they involve the lower lobes in 98% of cases. While extralobar sequestration is accepted as a congenital anomaly, substantial evidence suggests that intralobar sequestrations may have an acquired origin. Reports of intralobar sequestrations in infants are virtually absent and associated anomalies are relatively uncommon compared with their prevalence in association with other congenital thoracic anomalies (Stocker 1986, Frazier et al. 1997). Like an extralobar sequestration, accessory lung has its own pleural covering. It also has a rudimentary bronchus, which may be patent and communicate with the trachea, the tracheal bifurcation, or the esophagus. Fifty percent of extralobar sequestrations present in the first 6 months of infancy. Clinical manifestations include dyspnea and cyanosis. The frequent association of other congenital anomalies also may lead to their detection. Intralobar sequestrations present with productive cough and recurrent episodes of pneumonia. Up to 50% of patients will have become symptomatic by the age of 20 years. The characteristic appearance of an extralobar sequestration is that of a well-defined round, oval, or triangular opacity located posteromedially at the lung base. Intralobar sequestrations are more variable in appearance. Findings include a well-defined homogeneous opacity, solitary pulmonary nodule, recurrent episodes of pneumonic consolidation, and an area of pulmonary hyperlucency. Infection with fistula formation to an adjacent bronchus may lead to formation of a multilocular cystic mass with air-fluid levels. This “cystic” transformation of an initially homogeneous mass in the left vertebrophrenic angle is strongly suggestive of the correct diagnosis (Fig. 2.1). Extralobar sequestrations may appear homogeneous and echogenic. If an intralobar sequestration becomes infected, a multicystic mass may be seen. CT findings include a solid mass in both intra- and extralobar sequestrations. In addition, an area of homogeneous consolidation or a complex cystic mass may be seen in the intralobar variant. A peripheral zone of decreased lung attenuation is sometimes seen in intralobar sequestrations (Fig. 2.2) and has been attributed to collateral air drift through the incomplete, partially fibrous boundary between sequestrated and normal lung (Scully et al. 1981). Fig. 2.2a–c Intralobar sequestration. Axial CT image (a) shows a lobulated mass with a surrounding rim of decreased lung attenuation in the left lower lobe. Maximum intensity projection (MIP) reconstructions from CTA study show the systemic arterial supply from the thoracic aorta (b) and pulmonary venous drainage to the left atrium (c). Occasionally, a focal area of decreased lung attenuation in the lower lobes may be the only parenchymal manifestation of intralobar sequestration (Ikezoe et al. 1990). Dedicated thin-collimation helical CT with intravenous contrast medium allows demonstration of the systemic arterial supply and pulmonary/systemic venous drainage (Fig. 2.3) Fig. 2.3a, b Intralobar sequestration. CT shows a large area of decreased attenuation in the left lower lobe with a small central tubular opacity (a). Shaded surface display (SSD) 3D reconstruction demonstrates the systemic arterial supply and pulmonary venous drainage (b). Fig. 2.4a–c Intralobar sequestration. CT shows a partly cystic lung lesion (a) which is supplied by a branch of the aorta and drained by pulmonary venous branches as seen at angiography (b, c). Pulmonary arteries (white) and pulmonary veins (black) as seen on DSA study (c). CT has become the imaging modality of choice in investigation of sequestrations, and conventional angiography now is performed much less frequently. Aortography demonstrates a systemic arterial supply to the sequestration from the thoracic aorta in 70% of cases, from the abdominal aorta in 20%, and from an intercostal artery in 5% (Ranniger and Valvassori 1964). An intralobar sequestration drains to the pulmonary veins; an extralobar sequestration drains to the vena cava, hemiazygos vein, azygos vein, or portal vein (Fig. 2.4). Hypogenetic lung syndrome almost always is seen on the right side. The right lung is hypoplastic, and both the right bronchial tree and pulmonary vessels show incomplete development. The hypoplastic lung therefore derives a significant proportion of its perfusion from the systemic circulation via branches of the aorta. The anomalous pulmonary vein runs inferiorly through the lung before curving medially to enter the inferior vena cava (IVC); it has the shape of a scimitar, thus accounting for the name of the syndrome. Congenital cardiac defects, usually septal defects, are present in 25% of cases, and some patients also have bronchiectasis and tracheobronchial anomalies. Clinically, patients may be asymptomatic or may suffer from recurrent infections. A significant left-toright shunt may give rise to exertional dyspnea. The chest radiograph may show a small right lung and cardiac dextroposition. The “scimitar” vein descends vertically and then curves medially to join the IVC (Fig. 2.5). CT confirms the diagnosis and shows the scimitar vein draining to the IVC (Fig. 2.6) and the hypoplastic right pulmonary artery. It also may demonstrate associated bronchiectasis and tracheobronchial anomalies. Fig. 2.5a, b Scimitar syndrome. The right lung is hypoplastic and there is ipsilateral cardiac displacement. The right lower lobe vein drains to the vena cava (scimitar pattern), and the right upper lobe vein drains to the azygos vein. Fig. 2.6a–c Scimitar CXR and CT. Chest radiograph (a) shows scimitar vein. Axial CT images (b and c) show vein traversing the right lower lobe and draining into the inferior vena cava.
Bronchopulmonary Sequestrations
Pathology
Extralobar Sequestration
Intralobar Sequestration
Accessory Lung
Clinical Features
Radiologic Findings
Chest Radiograph
Sonography
Computed Tomography (CT)
Angiography
Hypogenetic Lung Syndrome (Scimitar Syndrome)
Clinical Features
Radiologic Findings
Bronchogenic Cysts
Pathology
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree