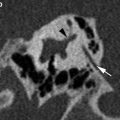
Fig. 1
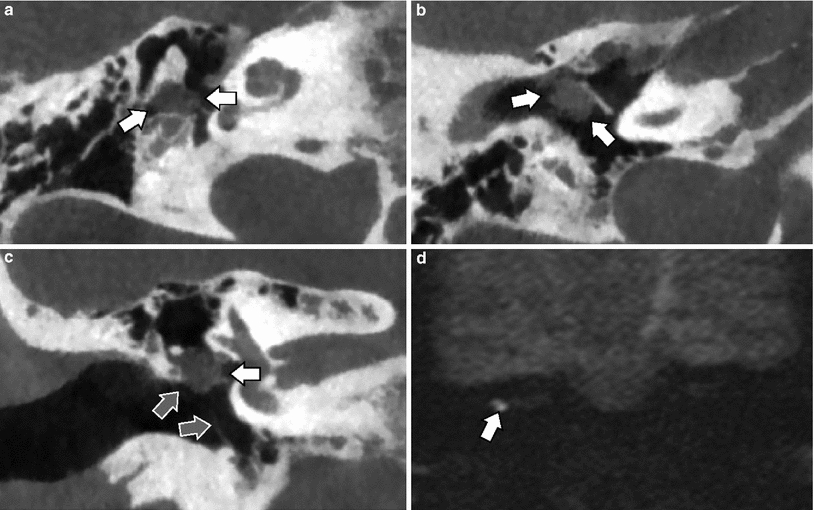
Cone-Beam CT—double-oblique reformatted images. a On axial images, the incudostapedial axis is angulated 15–25° posteriorly with regard to the coronal plane. Images must be reformatted along this line, passing through the joint and between the two crurae of the stapes (black line). Note the false appearance of a thickened footplate seen as high density between the grey fluid in the labyrinth and the black air in the middle ear. b On the oblique reformatted image (made along the black line in a), the incudostapedial joint is now angulated 20–30° superiorly with regard to the axial plane. Images must be reformatted along this line, passing through the joint and the centre of the oval window (white line). Note that the footplate no longer seems to be thickened. c On this double-oblique reformatted image (made along the black line in a and white line in b) one can see the joint, the crurae (white arrow) and the footplate (black arrow) in one plane. The footplate is normal and the fluid is in contact with the air without any bone density in between. Compare with “a” where this was not the case and the density between the fluid and air represented partial volume through the bone around the oval window/footplate
Magnetic resonance (MR) imaging has become the primary imaging technique for screening of congenital inner ear malformations. The imaging quality increased with the advent of better phased-array coils, a higher main magnetic field and stronger gradients. Three-dimensional (3D) heavily T2-weighted images provide excellent contrast between the high-signal intensity of cerebrospinal fluid (CSF), endolymphatic and perilymphatic fluid and the low-signal intensity surrounding osseous structures of the temporal bone. Similar images allow separate visualization of the three branches of the VIIIth nerve and the facial nerve inside the internal auditory canal (IAC). At 3.0 Tesla, the signal-to-noise is good enough to acquire submillimetric (isotropic 0.33–0.43 mm) images with enough contrast resolution and anatomic detail, even when a routine phased-array head coil is used. At 1.5 Tesla, phased-array surface coils are often needed to compensate for the lower signal-to-noise ratio (Schmalbrock et al. 1995). Three-dimensional Fourier transform constructive interference in steady state (3DFT-CISS), 3D turbo spin-echo (3D-TSE), 3D fast imaging employing steady-state acquisition (3D-FIESTA) and 3D driven equilibrium radio frequency reset pulse (3D-DRIVE) are some of the best known heavily T2-weighted sequences used to study the inner ear (Brogan et al. 1991; Casselman et al. 1993a, b, 1996; Schmalbrock et al. 1993; Tien et al. 1993). Even small anatomic structures like the utricular macula and the ganglion of Scarpa can be depicted on these high-quality images. Subtle congenital inner ear malformations like modiolar anomalies, incomplete partition anomalies of the cochlea and hypoplasia or aplasia of the cochleovestibular nerve or its cochlear branch can all be recognised on these images. They can also be used to produce 3D-maximum intensity projections (3D-MIP) and 3D-virtual reality (VR) surface rendered images. They are very helpful in the analysis of complex malformations which are difficult to evaluate on routine orthogonal images. T1-weighted images lack this good contrast between bone, nerves and fluid. Nevertheless, gadolinium-enhanced T1-weighted images remain the most sensitive images if one wants to exclude concomitant tumoral and inflammatory changes in adult patients with congenital sensorineural hearing loss (SNHL). The diagnosis of a congenital cholesteatoma can be made on CT when the middle ear is normally aerated. However, it can be very difficult to depict a congenital cholesteatoma on CT when a middle ear effusion is present. In these cases, non-echo-planar diffusion-weighted imaging (non-EPI DWI) with a b-value of 1,000 (b = 1,000 s/mm2) can be used to distinguish the cholesteatoma from the middle ear effusion (De Foer et al. 2010).
2 Congenital Malformations of theExternal Auditory Canal , Tympanic Membrane and Ring
2.1 Embryology
By about 4–5 weeks of fetal age, the first branchial groove deepens to become the primitive external auditory meatus. The first pharyngeal pouch which arises from endoderm of the primitive foregut grows outward and upward and will lead to the development of the primitive eustachian tube. For a short time, the epithelium of the first branchial groove and the endoderm of the first pharyngeal pouch will be in contact at the site where the future tympanic membrane will develop. During the next 20 fetal weeks, the first branchial groove contributes to the development of the mature external auditory canal, the outer layer of the tympanic membrane and the tympanic ring . The eustachian tube and middle ear cavity and its epithelial lining develop from the first pharyngeal pouch.
At 8 weeks, inward extension of the first branchial groove in the direction of the middle ear creates a primitive canal between the first and second branchial arches. At 9 weeks, the “meatal plug”, a solid cord of ectoderm grows from the fundus of the primitive EAC towards the middle ear. Ingrowth of mesoderm between the meatal plug and the middle ear forms the central fibrous portion of the tympanic membrane and explains why the mature tympanic membrane has an outer ectodermal (squamous epithelium), middle mesodermal (fibrous) and inner endodermal (respiratory epithelium) layer. At the same time, the first ossification centre of the bony tympanic ring appears. Disintegration and canalization of the meatal plug, which starts in the deepest part of the meatal plug near the tympanic membrane, will result in the formation of the bony part of the EAC. Failure of development of the first branchial groove resulting in non-formation of the primitive auditory canal or incomplete or absent canalization of the meatal plug will result in a spectrum of EAC, tympanic membrane and the tympanic ring malformations ranging from minor EAC stenosis to severe EAC atresia . Normal growth of the temporomandibular joint (TMJ) and middle ear also depends on the presence of a normal meatus and tympanic ring development. Congenital middle ear cholesteatomas can also develop when ectodermal tissue rests remain in the middle ear during fetal gestation.
The cartilaginous and osseous portions of the EAC develop from the first branchial arch mesoderm. The cartilages from the first and second branchial arch also form the ossicles as well as the mandible and midface. Hence ossicular anomalies, especially ossicular fusion, and more severe facial and mandibular deformities can occur together with EAC atresia. Unlike the rest of the ossicles, the anterior process of the malleus differentiates from the primitive tympanic ring. This explains why the malleus is frequently fixed to the atretic plate at this site (Dayal 1973; Bellucci 1981; Schuknecht 1989; Curtin et al. 2011).
2.2 Stenosis and Atresia of the External Auditory Canal
Stenosis or atresia of the EAC occurs once per 10,000–20,000 births and is most frequently unilateral. (Jafek et al. 1975). Its occurrence is sporadic due to spontaneous chromosomal abnormalities, which can be caused by infections or toxicity during gestation. However, genetic transmission explains the association of EAC atresia and many syndromes: hemifacial microsomia, Treacher Collins syndrome, Branchio-Oto-Renal syndrome (BOR), Crouzon or Alport syndrome, Klippel-Feil anomaly etc. (Romo et al. 2011). These patients present with conductive hearing loss.
2.2.1 Stenosis of the External Auditory Canal
The cartilaginous portion of the EAC can be stenotic, short or its course can be abnormal. The bony external auditory canal is considered stenotic when its diameter is smaller than 4 mm. Stenosis can also be associated with middle ear and ossicular abnormalities, but this association is more frequently seen in case of atresia (Fig. 2). Epithelial debris gets entrapped in the medial part of the EAC once the diameter of the EAC is smaller than 2–3 mm. These patients are prone to the development of an acquired EAC cholesteatoma (Cole and Jarsdoerfer 1990; Benton and Bellet 2000).
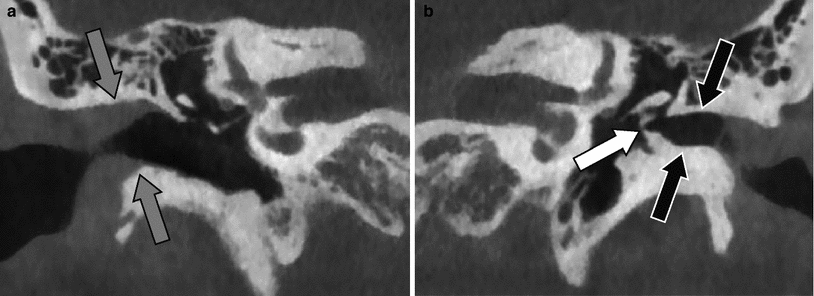

Fig. 2
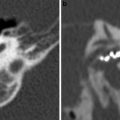
Stenosis of the external auditory canal. a Coronal CBCT image on the right side with normal size of the external auditory canal (grey arrows). b Coronal CBCT image on the left side stenosis of the bony part of the external auditory canal (black arrows). Fixation of the malleus to the tympanic ring (white arrow). There was no fibrous or bony atresia in this patient
2.2.2 Atresia of the External Auditory Canal
Atresia of the EAC can be membranous or bony. In “membranous atresia ”, the bony EAC is usually stenotic and a soft tissue mass is occupying the region where the tympanic membrane normally is located. Associated malformations of the ossicles and middle ear are less frequent in case of membranous atresia (Fig. 3).
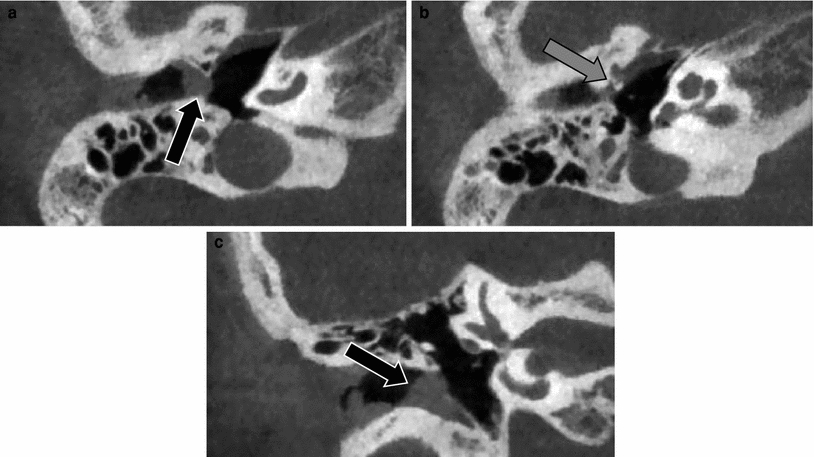

Fig. 3
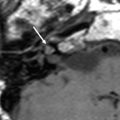
Membranous atresia of the external auditory canal—Jahrsdoerfer Type B. a Axial CBCT image through the EAC shows a soft tissue mass at the site where the tympanic membrane is normally situated (black arrow). b Axial CBCT image made more cranial than image a. The anterior process of the malleus, which differentiates unlike the rest of the ossicles from the primitive tympanic ring, is fixed to the tympanic ring (grey arrow). c Coronal CBCT image through the EAC. Membranous atresia with soft tissues at the site of the tympanic membrane (black arrow)
In “bony atresia ” of the EAC, the tympanic membrane is replaced by a bony plate of variable thickness, extending in the EAC, however, this bony atresia can also be incomplete (Fig. 4).
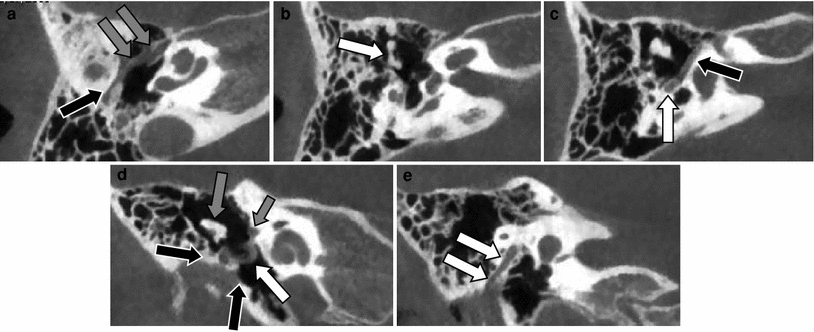
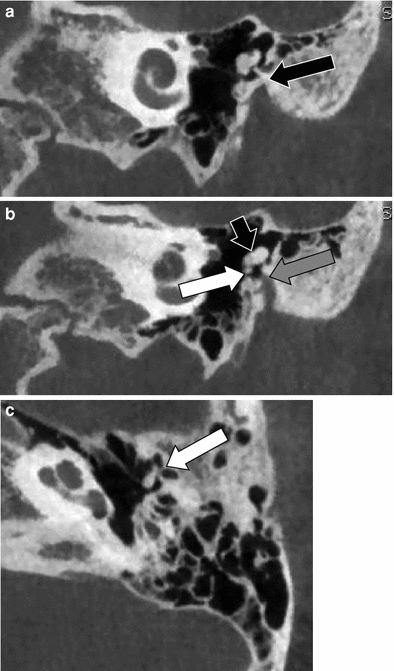

Fig. 4
Incomplete bony atresia of the EAC with abnormal course of the facial nerve and tensor tympani muscle. a Axial CBCT image at the level of the tensor tympani muscle shows how this muscles follows an abnormal course through the middle ear cavity (grey arrows) and ends at the bony atresia plate (black arrow). b Axial CBCT image at the level of the oval window shows a normal position and development of the stapes but the malleus and incus are malformed and fused (arrow). c Axial CBCT image at the level of the tympanic segment of the facial nerve canal (black arrow). The posterior genu and mastoid segment of the facial nerve are showing a slightly anterolateral displacement (white arrow). Notice that the articulation of the malleus and incus has an abnormal mediolateral orientation. d Coronal CBCT image through the anterior part of the middle ear shows the incomplete bony atresia (black arrows), the fused malleus and incus (grey arrow) and the abnormal course of the thickened tensor tympani muscle (white arrow). Tympanic segment of the facial nerve canal (small arrow). e Coronal CBCT image through the posterior part of the middle ear cavity showing the abnormal oblique lateral shift of the mastoid segment of the facial nerve canal (white arrows)

Fig. 5
Bony atresia—Jahrsdoerfer Type C. a Coronal CBCT image through the anterior part of the middle ear cavity. The tympanic membrane is replaced by a bony plate (black arrow). b Coronal CBCT image posterior to “a”. The bony atresia is not complete. There is still some membranous atresia and a very stenotic EAC filled with soft tissue can be seen (grey arrow). Notice the fixation of the anterior process of the malleus to the tympanic bony atresia plate (white arrow). The head of the malleus and body of the incus are fused (short arrow). c Axial CBCT image showing the fixation of the anterior process of the malleus to the bony atresia plate (white arrow)
In “complete bony atresia”, the EAC is absent and a bony atresia plate seals the lateral wall of the middle ear cavity. The surrounding skull base structures show a centripetal shift towards the area where the EAC should have been (Benton and Bellet 2000). Complete bony atresia is often associated with:
A higher and posterior displaced condylar fossa
An anterior displaced mastoid process
A shift of the condyle towards the mastoid process and localization lateral to the middle ear cavity
Absence of the ascending ramus of the mandible
A high jugular bulb and low tegmen
A dehiscent and/or inferior displaced tympanic segment of the facial nerve
An anterior and lateral displacement of the mastoid segment of the facial nerve (Figs. 4 and 6).
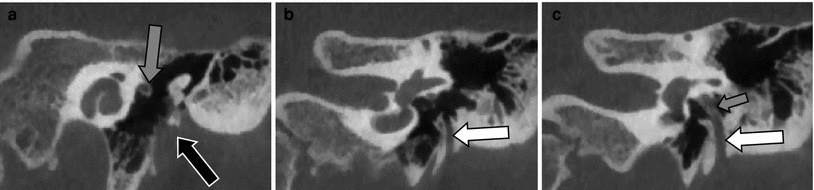
Fig. 6
Membranous atresia, abnormal course of the mastoid segment of the facial nerve. a Coronal CBCT image through the anterior part of the middle ear cavity showing the membranous atresia of the external auditory canal (black arrow) and the normal position of the tympanic segment of the facial nerve canal (grey arrow). b Coronal CBCT image posterior to (a) showing the anterolateral displacement of the mastoid segment of the facial nerve canal (white arrow). c Coronal CBCT image at the level of the round window showing the anterolateral displaced dehiscent mastoid segment of the facial nerve running through the middle ear cavity (short arrow) and through its anterolateral displaced canal (white arrow)
A simple and useful classification of EAC stenosis and atresia was proposed by Schuknecht and was modified by Benton and Jahrsdoerfer (Schuknecht 1989; Benton and Bellet 2000; Yeakley and Jahrsdoerfer 1996). It facilitates the communication between radiologists and otological surgeons and further helps to make the choice between surgery and placement of an osteo-integrated bone-conduction device (BAHA system) (Evans and Kazahaya 2007).
Type A or meatal atresia: The lateral fibrocartilaginous part of the canal is stenotic
Type B or partial atresia: The fibrocartilaginous and bony parts of the canal are narrowed and sometimes tortuous. The tympanic membrane is small. The manubrium of the malleus is frequently short and curved and the malleus may be fixed to the epitympanum or tympanic ring (Fig. 3)
Type C or total atresia: There is absence of the external auditory canal, a partial or complete atresia plate and a well-developed middle ear cavity are present. The malleus and incus are deformed and fused, the malleus neck is fused to the atretic plate (Fig. 5)
Type D or hypopneumatic total atresia. Similar to Type C but the middle ear cavity is underdeveloped and there is little or no mastoid pneumatization. The facial nerve is often aberrant (Fig. 7).
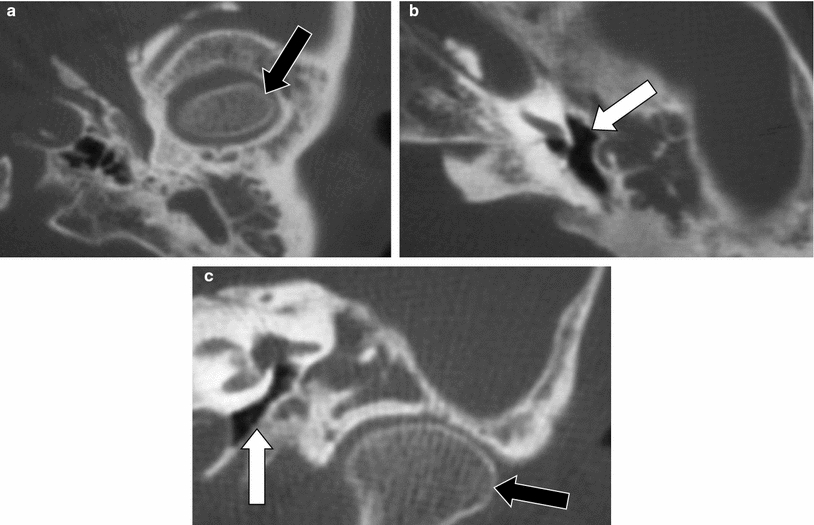
Fig. 7
Bony atresia with hypoplasia of the middle ear cavity—Jahrsdoerfer Type D. a Axial MDCT image through the mastoid showing the protrusion of the TMJ in the skull base (black arrow). b Axial MDCT image at the level of the round window: atresia of the EAC and extreme hypoplastic middle ear cavity (white arrow). c Coronal MDCT image through the TMJ showing the cranial displacement of the TMJ (black arrow) which is now situated lateral to the hypoplastic middle ear cavity (white arrow). Notice the absence of the ossicles
This classification already helps the radiologist to look carefully at the structures which could jeopardize surgical repair. The following findings make repair of the EAC and ossicular chain more difficult or impossible:
Mandibular condyle lateral to the middle ear cavity—difficult to reconstruct the EAC
Small middle ear cavity (distance promontory—atretic plate of less than 3 mm)
Closed or stenotic oval and/or round window
Abnormal course of the facial nerve interfering with the surgical approach
Absence of normal malleus-incus complex (severe deformity or dissociation), absence of the incudostapedial articulation, absence of the stapes suprastructure.
Finally, in severe aural atresia inner ear malformations can be present, although the inner ear develops completely separately from the EAC and middle ear. Hence, inner ear imaging is needed to exclude inner ear malformations in these patients.
2.2.3 First Branchial Cleft Anomalies
First branchial cleft anomalies occur when there is incomplete obliteration of the first branchial apparatus. Remnants of the first branchial cleft can then give rise to a first branchial cleft cyst, fistula or sinus and the majority of first branchial cleft remnants are cysts. In “Type I” first branchial cleft cysts (first BCC), which are of ectodermal origin, a soft and painless well-circumscribed cyst is found just anterior, inferior or posterior to the EAC and they can run parallel with the EAC and are often only related to the membranous EAC. However, the cyst can beak towards the bony-cartilaginous junction of the EAC in both Type I and Type II first BCCs. ““Type II” ” first BCCs are of ectodermal and mesodermal origin and can extend from the EAC to the angle of the mandible (Fig. 8) and can even reach the posterior submandibular space. They are found deep to—and even in the parapharyngeal space (Fig. 9), in or superficial to the anterior part of the parotid gland, anterior to the sternocleidomastoid muscle, superior to the hyoid bone and end at the bony-cartilaginous junction of the EAC. Typically, the wall of the cyst is not enhancing, enhancement of a thickened wall is only seen when the first BCC is infected. Complete surgical resection is needed to treat the associated recurrent periauricular abscess formation or chronic otorrhea caused by the sinus tract drainage. Good facial nerve exposure is needed during surgery because of the often close relation of the first BCC with the facial nerve (Lambert and Dodson 1996; Mukherji et al. 1993; Triglia et al. 1998; Work and Proctor 1963).
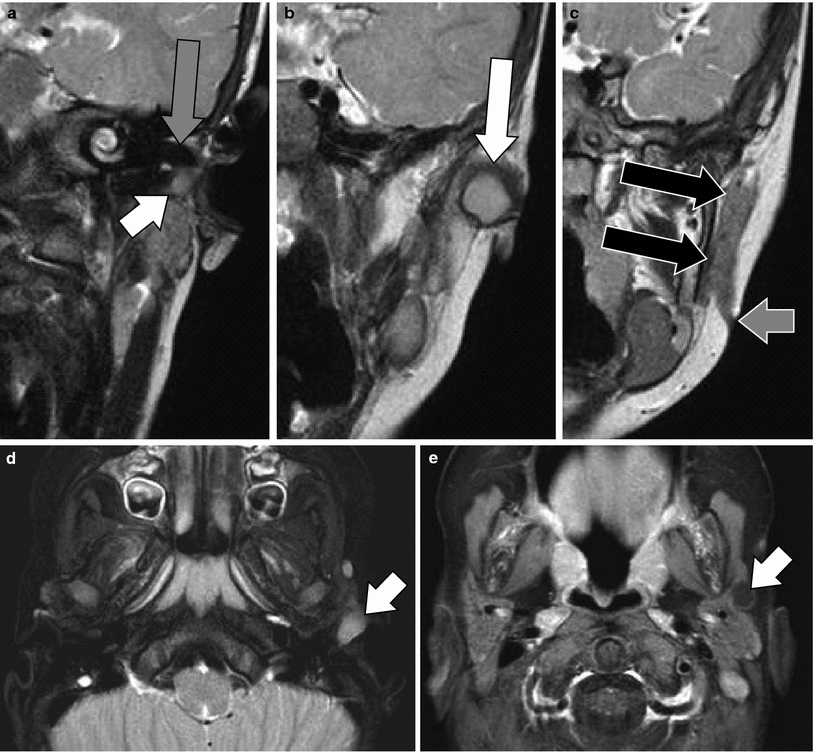
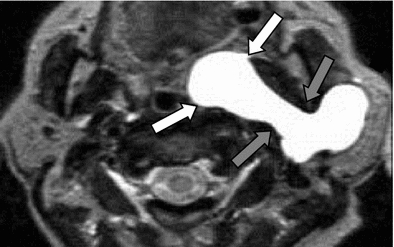

Fig. 8
Type II cyst and fistula of the first branchial cleft. a–c Coronal T2-weighted turbo spin-echo images through the external auditory canal (EAC) (a), the retromandibular region (b) and ascending ramus of the mandible (c). A cyst can be followed to the bony-cartilaginous junction of the floor of the EAC (small white arrow) and becomes larger in the region anterior to the EAC (large white arrow). A large fistula (black arrows) can be followed downward to the mandibular angle where the cutaneous opening of the fistula is seen (small grey arrow). Roof of the EAC (large grey arrow). d Axial T2-weighted image with fat suppression at the level of the floor of the EAC showing the high-signal intensity in the branchial cleft cyst (arrow). e Axial gadolinium-enhanced T1-weighted image with fat suppression at the level of the parotid gland demonstrating the first branchial cleft fistula along the anterolateral border of the parotid gland with subtle enhancement of the wall of the fistula (arrow)

Fig. 9
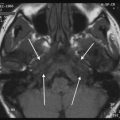
Type II cyst of the first branchial cleft with extension in the prestyloid parapharyngeal space. Axial T2-weighted image through the parotid glands showing a very rare extension of a first branchial cleft cyst deep to the left parotid gland, through the stylomandibular tunnel (grey arrows) into the prestyloid parapharyngeal space (white arrows)
3 Congenital Malformations of the Middle Ear
3.1 Embryology
At the third gestational week, an outpouching of the endoderm-lined first pharyngeal pouch, will form the tympanic cavity and auditory tube. This outpouching of the foregut, the tubotympanic recess, grows dorsally between the otic capsule and primitive external auditory canal. Progressive resorption of the mesenchyma surrounding the primary middle ear cavity will lead by the end of the eight gestational week to cavitation and expansion of the tympanic cavity. Remnants of the mesenchyma eventually form the ligaments which will support the middle ear ossicles. The mesoderm of the first and second branchial arch forms the remaining middle ear structures being the ossicles, muscles and tendons. Meckel’s cartilage , the cartilage anlage of the first branchial arch, forms the head and neck of the malleus, the malleal ligaments, the body and short process of the incus and the tensor tympani muscle. The tensor tympani muscle is innervated by the mandibular nerve, the nerve of the first branchial arch. Reichert’s cartilage , the cartilage anlage of the second branchial arch, forms the manubrium of the malleus, the long process of the incus, the capitulum, crura and the larger central part of the footplate (Thompson et al. 2012) and the stapedius muscle which is innervated by the facial nerve, the nerve of the second branchial arch. As already mentioned, the anterior process of the malleus differentiates from the primitive tympanic ring unlike the rest of the ossicles. During the 5th–6th week of gestation, the stapes, derived from the second branchial arch, develops around the stapedial artery, explaining the ring-like configuration of the stapes. The stapedial ring will further enlarge and the mesenchymal tissue will differentiate into cartilage. The stapes will reach the otic capsule at the region of the future oval window by 4 months and the area of fusion is the dense fibrous tissue of the oval window that forms at the end of the fissure between primordial otic and basioccipital ossification centers (Thompson et al. 2012, Clack 2002) and will become the medial vestibular surface of the footplate. (Arey and Real 1974; Anson et al. 1991; Williams 1992).
3.2 Middle Ear Malformations Associated with External Auditory Canal Deformities
These malformations were described and discussed above (see “Atresia of the External Auditory Canal”).
3.3 Middle Ear Malformations in the Absence of EAC Stenosis and EAC Atresia
3.3.1 Isolated Ossicular Deformities
Isolated ossicular deformities occur in 38 % of the congenital malformations of the external and/or middle ear and are bilateral in half of the cases (Anson and Donaldson 1981; Swartz and Faerber 1985; Sando et al. 1988; Nager 1993). Anomalies of the middle ear and ossicles without EAC stenosis and EAC atresia are much less common than outer ear anomalies. The stapes and the incus are the most frequently affected ossicles. These ossicular anomalies can be inherited but association with syndromes, especially with Goldenhar’s and Treacher Collins syndromes, is known. Isolated maldevelopment of the first branchial arch (Meckel’s cartilage ) will cause anomalies of the neck and head of the malleus, and of the body and short process of the incus. The mandible is also formed by Meckel’s cartilage and hence mandibular and first branchial arch ossicular malformations are often associated. Maldevelopment of the second branchial arch (Reichert’s cartilage ) will cause anomalies of the manubrium of the malleus, long process of the incus, stapes superstructure and lateral surface of the footplate of the stapes, styloid process and facial nerve canal. Maldevelopment of both the first and second branchial arch can of course result in complete absence of one or more of the ossicles. The stapes is the most frequently involved ossicle and a broad spectrum of anomalies can be found: footplate fixation, hypoplasia of the stapes, subtle abnormalities of the crura, monopodal stapes and in the worst case absence of the complete stapes (Fig. 10). Subtle anomalies of the footplate were only rarely reported in the past, but with the advent of high-resolution CT and CBCT and the use of double-oblique reconstructions, these anomalies are more frequently reported (Fig. 11). Incus aplasia, long process malformations, absent incudostapedial joint or fibrous appearance of this joint are some of the most frequent anomalies of the incus (Figs. 12 and 13). Anomalies of the malleus are: aplasia, disappearance of the joint with the incus and fusion with the incus (Fig. 14), fusion of the manubrium with the long process of the incus and the capitulum of the stapes and fixation of the head of the malleus to the epitympanic wall with a “malleus bar” (Fig. 15). Detailed imaging of the facial nerve, oval window and middle ear cavity itself is required whenever surgical reconstruction of the malformed ossicular chain is considered.
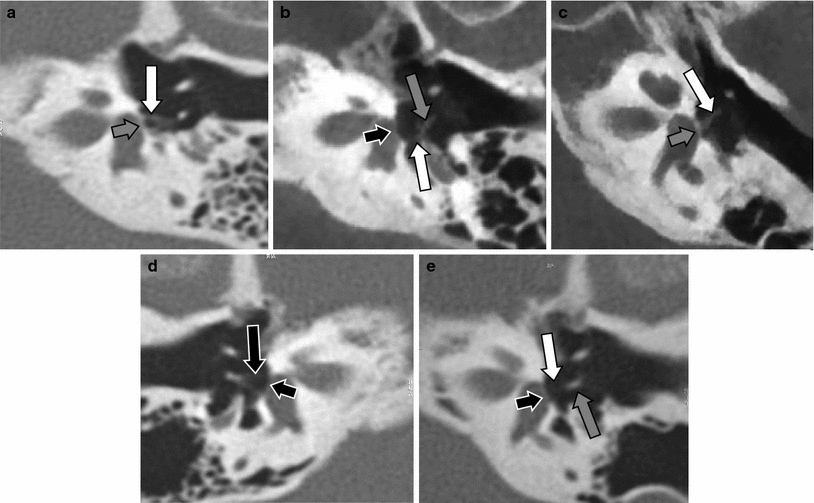
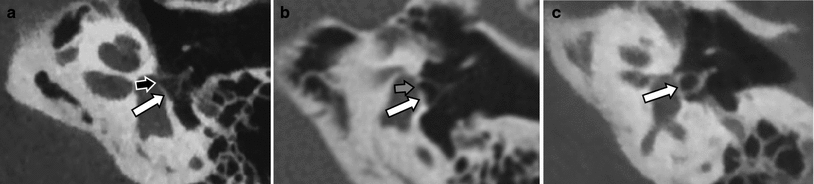
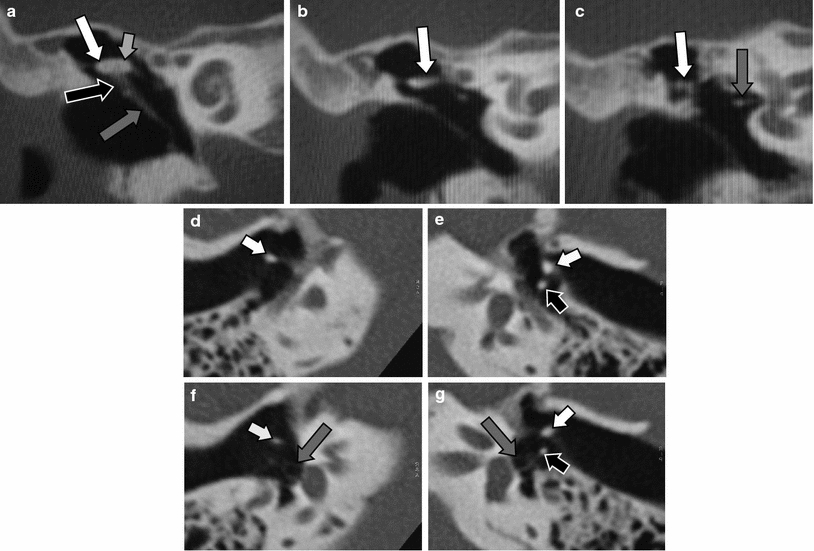
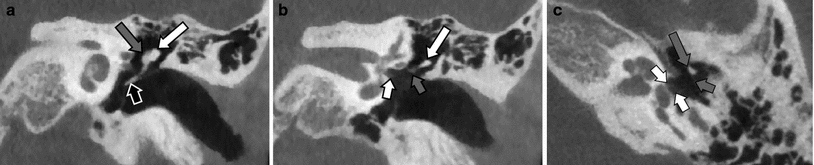
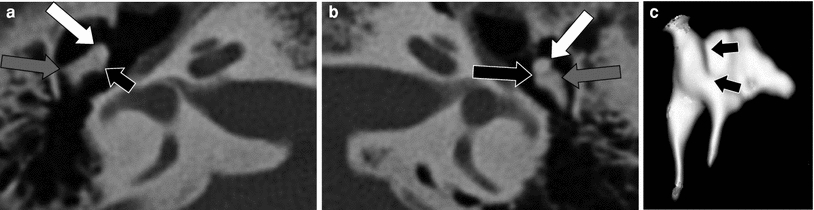
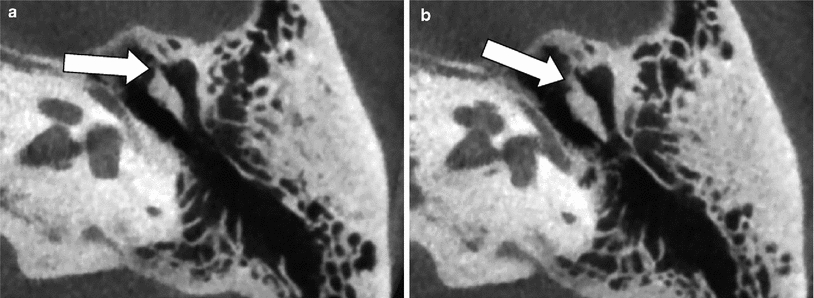

Fig. 10
Isolated congenital stapes deformities. a Double-oblique reformatted MDCT image of the left middle ear in a patient with a hypoplastic stapes (white arrow), compare with a normal-size stapes in Figs. 1c and 10d. Abnormal footplate (grey arrow). b Double-oblique reformatted CBCT image of the left middle ear in a patient with a stapes with only a posterior crus (white arrow), the anterior crus was absent. Capitulum of the stapes (grey arrow), normal footplate without any calcification located between the fluid in the vestibule and air in the middle ear (black arrow). c Double-oblique reformatted CBCT image of the left middle ear in a patient with a monopodal stapes (white arrow) fixed on the centre of a thickened and calcified footplate (grey arrow). This deformity was present on both sides in this patient. d–e Double-oblique reformatted MDCT image of the right (d) and left (e) middle ear in a patient with aplasia of the left stapes (white arrow). Normal stapes on the right side (black arrow), lenticular process of the incus on the left side (grey arrow), normal footplate (small black arrows)

Fig. 11
Congenital footplate thickening/oval window closure in patients with a normal stapes suprastructure and normal course of the facial nerve and who presented with congenital conductive hearing loss. a Double-oblique reformatted CBCT image in a patient with an almost completely calcified and closed footplate/oval window (white arrow) and a remaining subtle opening at the anterior border of the footplate (black arrow). Non-ossified hypodense stapes suprastructure. b Double-oblique reformatted MDCT image in a patient with a remaining central opening in the footplate/oval window (grey arrow) located between a smaller anterior and larger posterior (white arrow) calcified footplate plaque. Non-ossified hypodense stapes suprastructure. c Double-oblique reformatted CBCT image in a patient with a completely closed and calcified footplate/oval window (white arrow). Ossified hyperdense stapes suprastructure

Fig. 12
Aplasia of the long process of the incus. a Coronal MDCT image through the normal malleus: malleus head (small white arrow), neck of the malleus (black arrow), handle of the malleus (grey arrow) and corpus of the incus (large white arrow). b Coronal MDCT image through the incus: incus body (white arrow). c Coronal MDCT image through the stapes: short process of the incus (white arrow), stapes (grey arrow). d–g Axial MDCT images at the level of the long process of the incus (d, e) and incudostapedial joint (f, g). The long process of the incus can be seen on the left side (black arrows) but is absent on the right side, aplasia. Neck and handle of the malleus (white arrows), normal stapes (grey arrows)

Fig. 13
Aplasia (bilateral) of the long process of the incus in a patient with Treacher Collins syndrome. a Coronal CBCT image through the malleus. Normal head of the malleus (grey arrow), handle of the malleus (black arrow) and body of the incus (white arrow). b Coronal CBCT image through the body of the incus (white arrow). The long process of the incus is absent and is partially replaced by a fibrous structure (grey arrow). Normal stapes in the oval window niche (small white arrow). c Axial CBCT image through the stapes (white arrows). The normal ossified neck of the malleus can be seen (large grey arrow), the long process of the incus is absent and is replaced by a hardly visible fibrous structure (small grey arrow)

Fig. 14
Incudomallear fusion. a–b Axial MDCT images through the right (a) and left (b) middle ear. Head of the malleus (white arrows), corpus of the incus (grey arrows) with a slightly malformed body of the incus on the right side. Normal incudomallear joint on the left side (black arrow), absent joint on the right side as the head of the malleus and the corpus of the incus are fused (small black arrow). c 3D surface rendering of the right malleus and incus: absence of the joint between the head of the malleus and the corpus of the incus (black arrows)

Fig. 15
Congenital fixation of the head of the malleus to the anterior wall of the middle ear cavity—mallear bar. a–b Axial MDCT images through the upper and lower part of the head of the malleus on the left side. A calcified bridge (white arrows) is seen between the head of the malleus and the anterior wall of the middle ear cavity, reducing the mobility of the incus: mallear bar
3.3.2 Facial Nerve, Oval Window and Round Window Anomalies
Both the facial nerve and part of the ossicles develop from the second branchial arch. This explains why the facial nerve often has an anomalous course in the presence of ossicular malformations. In patients with congenital middle ear malformations and a normal EAC, the tympanic segment of the facial nerve can run over the oval window, can be found below the stapes under the oval window and sometimes lies on the stapes (Fig. 16). An abnormally wide Obtuse angle of the first or anterior genu of the facial nerve canal can also be present.
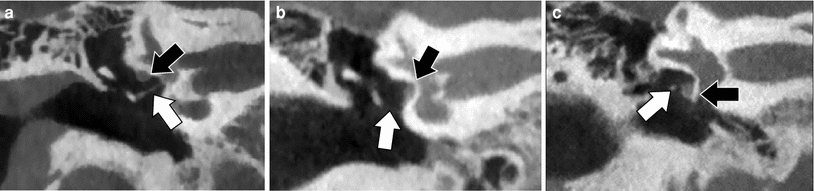

Fig. 16
Abnormal course of the tympanic segment of the facial nerve. a Coronal CBCT image through a normal right middle ear illustrating the normal position of the tympanic segment of the facial nerve canal under the lateral semicircular canal (black arrow) and above the stapes (white arrow). Notice the normal thickness of the footplate. b Coronal CBCT of the right middle ear. The facial nerve (black arrow) is lying on the surface of the closed/not developed oval window. The stapes cannot be depicted: aplasia of the stapes (white arrow). c Coronal CBCT of the right middle ear. The facial nerve (black arrow) is lying on the promontory below the oval window niche and the stapes (white arrow) can be seen above the facial nerve but below the completely closed or not formed oval window
Congenital malformation of the oval window is an associated finding in patients with a congenital abnormal course of the facial nerve. The hypothesis is that the abnormal position of the facial nerve prevents the stapes to make contact with the developing otic capsule at the site of the future oval window. Consequently, the development of the oval window is not induced. More subtle oval window anomalies like hypoplasia or partial absence of the oval window or thickening of the footplate could be caused by less important facial nerve hinderance (Fig. 17). These more subtle oval window anomalies and the abnormal course of the facial nerve can today be detected on high-resolution CT/CBCT images. However, the footplate of the stapes can only be evaluated in a reliable way when double-oblique reconstructions are made, otherwise subtle stapes and especially footplate anomalies may remain undetected (Jahrsdoerfer 1981; 1988; Zeifer et al. 2000; Miura et al. 2003; Vercruysse et al. 2006).
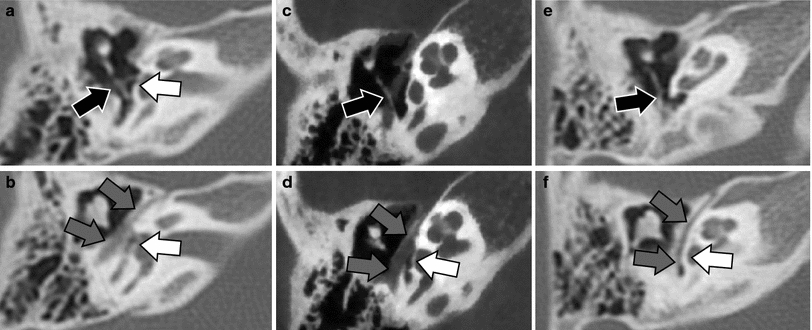

Fig. 17
Congenital absence or hypoplasia of the oval window. Axial MDCT (a, b, e, f) and CBCT (c, d) images at the level of the inferiorly displaced stapes (a, c, e) and at the level of the tympanic segment of the facial nerve canal and aplastic or hypoplastic oval window (b, d, f). a, b The stapes is displaced inferiorly and slightly posteriorly and is not longer exactly centred on the oval window area (black arrow). The oval window is extremely hypoplastic and only a small opening can be seen (white arrow). The tympanic segment of the facial nerve canal is positioned lateral to the hypoplastic oval window (grey arrows). c, d The stapes completely missed its contact with the oval window and is displaced inferoposteriorly in the sinus tympani (black arrow). The oval window is absent (white arrow) and the tympanic segment of the facial nerve canal (grey arrows) passes just lateral of the place where the oval window should have been formed. e, f The stapes again completely missed the contact with the oval window area and is displaced inferoposteriorly in the sinus tympani (black arrow). The oval window is not formed (white arrow) and the tympanic segment of the facial nerve is lying on the surface of the labyrinth at the site where the oval window should have developed (grey arrows). Images 17 c, d Courtesy Dr. Sebastien Janssens de Varebeke, ENT Department, Virga Jesse Hospital, Hasselt, Belgium
Absence of the round window is rare and is bilateral in nearly 50 % of the cases (Fig. 18), and is most frequently not associated with oval window anomalies. Stenosis of the round window and/or round window recess is more frequently found and the diagnosis is made when the round window or round window recess measures less than 1.5 mm. This is best measured in the sagittal plane (Veillon et al. 2014).
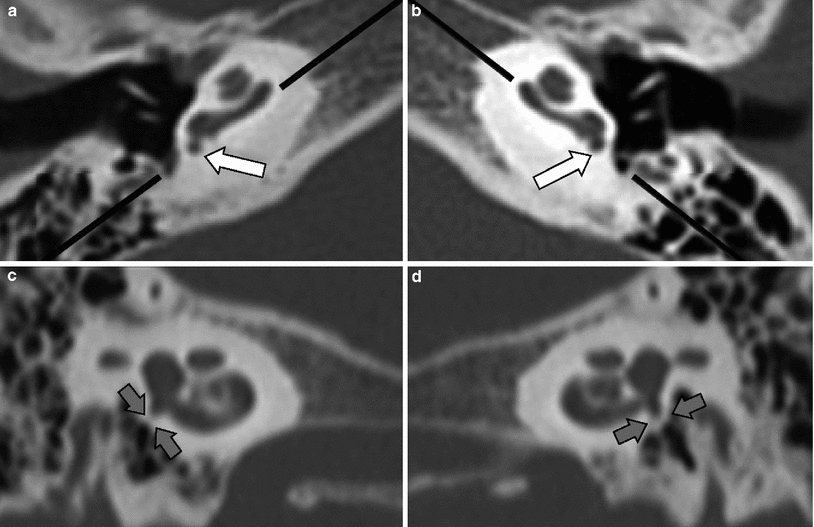

Fig. 18
Bilateral absence of the round window. Images of the right (a, c) and left (b, d) round window made in the axial (a, b) and parasagittal plane (c, d), the latter made along the black lines on the axial images. a, b The round window and round window recess are completely closed (white arrows). c, d The complete absence of the round window and round window recess is confirmed on the parasagittal images (grey arrows)
3.3.3 Other Middle Ear Anomalies and Congenital Middle Ear Cholesteatoma
The middle ear cavity can be hypoplastic (Fig. 7) which can also jeopardize surgical reconstruction. Absence of the stapedius muscle and/or tendon, absence or elongation of the pyramid eminence, abnormal course of the tensor tympani tendon (Figs. 4 and 19), absence of a normal cochleariform process, etc. are some of the other congenital anomalies which can be found in the middle ear. Congenital cholesteatomas account for 2 % of all middle ear cholesteatomas and can also be found in the middle ear cavity in the absence of EAC stenosis and EAC atresia. They are the result of misplaced embryonic rests of squamous cell epithelium (ectoderm) in the middle ear cavity (endoderm) during the development of the middle ear. In these cases, the tympanic membrane is completely normal and often there are no other signs of middle ear inflammation. These congenital cholesteatomas are most frequently located in the vicinity of the tensor tympani muscle canal and medial to the ossicles while acquired cholesteatomas are most often found lateral to the ossicles (Fig. 20). These congenital cholesteatomas have a convex shape. They are detected at the age of 4–5 years and are more frequent in boys (Levenson et al. 1989; Mc Gill et al. 1999).