Fibrosarcoma and Malignant Fibrous Histiocytoma
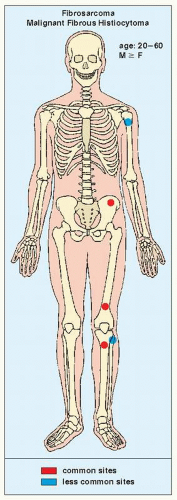
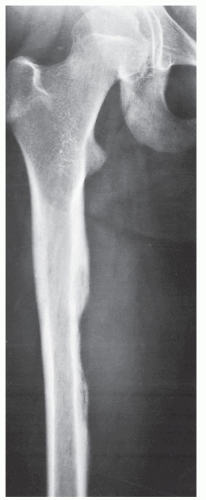
Fibrosarcoma and malignant fibrous histiocytoma (MFH) are malignant fibrogenic tumors that have very similar radiographic presentations and histologic patterns. Both typically occur in the third to sixth decades, and both have a predilection for the pelvis, femur, humerus, and tibia (
Fig. 22.1).
Because there is no essential difference in the imaging features, clinical behavior, and survival data for these tumors, it is justified to regard them as a single group. Both fibrosarcoma and MFH can be either primary tumors or secondary to a preexisting benign condition, such as Paget disease, fibrous dysplasia, bone infarct, or chronic draining sinuses of osteomyelitis. These lesions may also arise in bones that were previously irradiated. Such lesions are termed secondary fibrosarcomas (or secondary malignant fibrous histiocytomas). Rarely, fibrosarcoma can arise in a periosteal location (periosteal fibrosarcoma). Some investigators postulate, however, that in this location these lesions represent primary soft-tissue tumors abutting the bone and invading the underlying periosteum.
Histologically, fibrosarcoma and MFH are characterized by tumor cells that produce collagen fibers. In fibrosarcoma, however, there is a herringbone pattern of fibrous growth with mild cellular pleomorphism, whereas histiocytic features of a characteristic storiform or pinwheel arrangement of fibrogenic tissue typify MFH. In addition, numerous large bizarre polyhedral cells (histiocytic component) are present. Neither tumor is capable of producing osteoid matrix or bone, a factor distinguishing them from osteosarcoma.
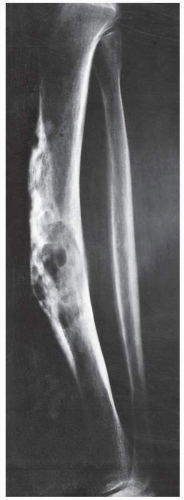
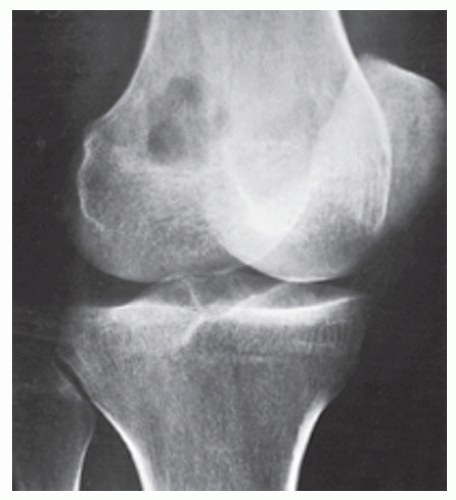
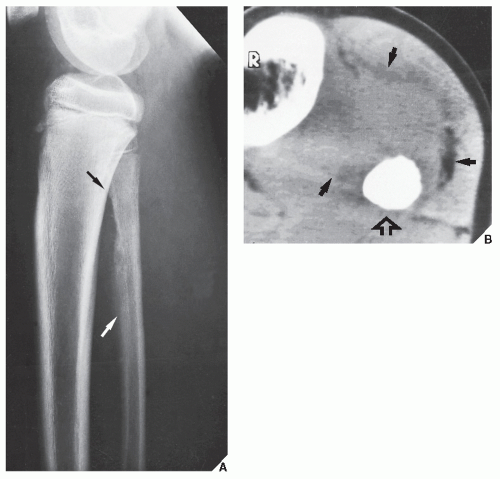
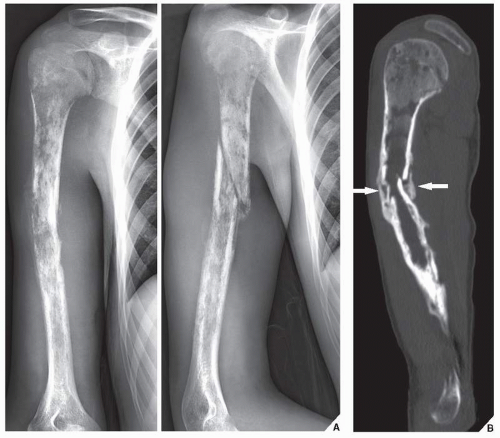
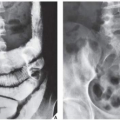
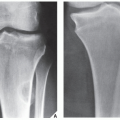
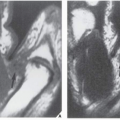
Radiographically, fibrosarcoma and MFH are recognized by an osteolytic area of bone destruction and a wide zone of transition; the lesions are usually eccentrically located close to or in the articular end of the bone. They exhibit little or no reactive sclerosis and in most cases, no periosteal reaction (
Figs. 22.2 and
22.3); a soft-tissue mass, however, is frequently present.
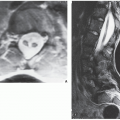
On computed tomography (CT) examination, fibrosarcoma and MFH show a predominant density similar to that of normal muscle and exhibit the nonspecific tissue attenuation values of Hounsfield units encountered in most nonmineralized tissues. Hypodense areas reflect areas of necrosis within tumor. Magnetic resonance imaging (MRI) is useful to outline the intraosseous and extraosseous extension of these tumors, but there are no characteristic MRI findings for either one (
Fig. 22.4). Some investigators found the signal characteristics comparable to those of other lytic bone tumors. Signal intensity is intermediate to low on T1-weighted images and high on T2 weighting, frequently heterogenous, and varying with the degree of necrosis and hemorrhage within the tumor.
It should be stressed, however, that the entity of MFH has recently fallen out of favor and into disrepute. For example, in the new World Health Organization (WHO) classification of soft-tissue tumors, MFH is considered to represent a small group of undifferentiated pleomorphic sarcomas with no definable line of differentiation, and the term is used with reluctance, although MFH of bone still remains in this classification listed under the heading “fibrohistiocytic tumors.”
Differential Diagnosis
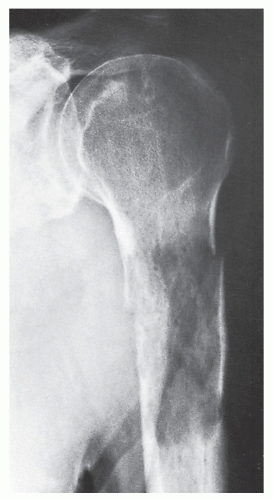
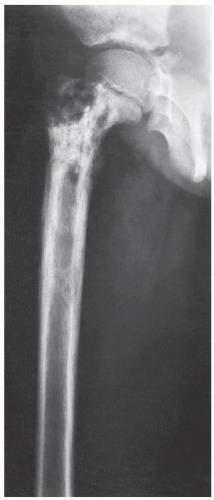
Fibrosarcoma and MFH may resemble a giant cell tumor (
Fig. 22.5) or telangiectatic osteosarcoma (see
Fig. 21.15). They are also often mistaken for metastatic lesions (see
Fig. 22.3). Some authorities believe that an almost pathognomonic sign of fibrosarcoma are small sequestrumlike fragments of cortical bone and spongy trabeculae, which may be demonstrated on conventional radiography or CT scan.
Immunohistochemical studies have been helpful in the diagnosis of MFH by demonstrating certain nonspecific markers of histiocytic enzymes such as lysozyme, α1-antitrypsin, and α1-antichymotrypsin in the tumor. Other antigens reported to variably stain MFH included vimentin, actin, desmin, and keratin.
Complications and Treatment
Because these tumors do not respond satisfactorily to radiation or chemotherapy, surgical resection is the treatment of choice. Pathologic fracture may occur and, as a palliative measure, internal splinting with a metallic implant may be justified. The tumor has been reported to recur after local excision and may spread to regional lymph nodes. As already stated previously, fibrosarcoma and MFH may complicate benign conditions such as fibrous dysplasia, Paget disease, bone infarction, or chronic draining sinuses of osteomyelitis. They may also arise in bones that were previously irradiated (see the discussion under the heading Benign Conditions with Malignant Potential). The 5-year survival rate after treatment varies according to different studies from 29% to 67%.
Ewing Sarcoma
Ewing sarcoma, a highly malignant neoplasm predominantly affecting children and adolescents, with decisive male predominance, is representative of the so-called round cell tumors. Its precise histogenesis is unknown, but it is generally thought that Ewing sarcoma originates from bone marrow cells. Some authorities, however, believe that Ewing sarcoma is a neurally derived small round cell malignancy very similar to the so-called primitive neuroectodermal tumor (PNET). Recent studies reveled that all tumors of the Ewing family are characterized by recurrent chromosomal translocations involving chromosomes 11 and 22 [t(11;22)(q24;q12)] or chromosomes 21 and 22 [t(21;22) (q22;q12)] in about 85% and 15% of cases, respectively. In about 20% of cases of Ewing sarcoma, the second most common genetic alteration is the inactivation of the gene
p16 or
INK4A. In particular,
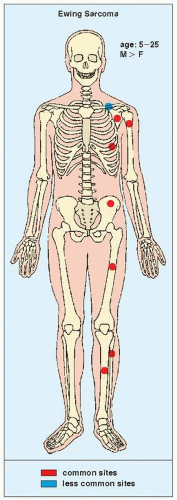
p16 deletions represent a significant negative predictive factor in Ewing tumor. Approximately 90% of Ewing sarcomas occur before age 25, and the disease is extremely rare in black persons. Ewing sarcoma has a predilection for the diaphysis of the long bones, as well as the ribs and flat bones such as the scapula and pelvis (
Fig. 22.6). Clinically, it may present as a localized painful mass or with systemic symptoms such as fever, malaise, weight loss, and an increased erythrocyte sedimentation rate. These systemic symptoms may lead to an erroneous diagnosis of osteomyelitis.
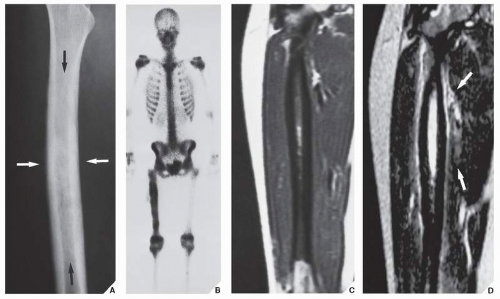
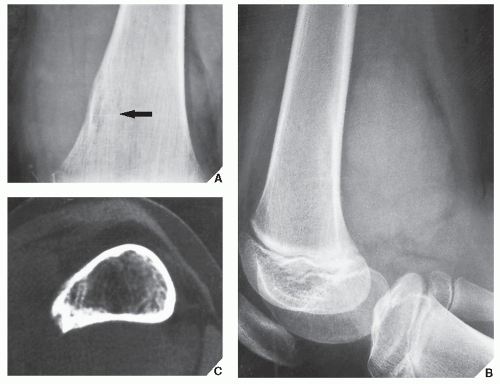
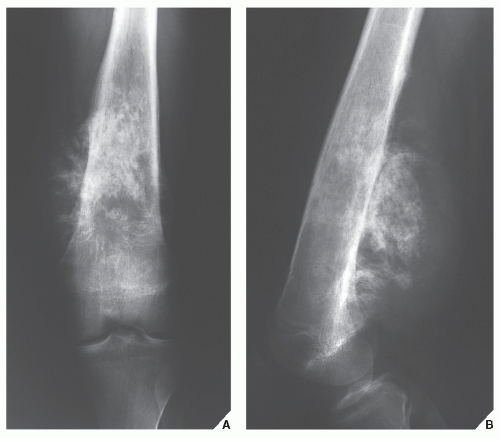
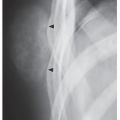
The imaging presentation of this malignancy is usually rather characteristic; the lesion is poorly defined, marked by a permeative or moth-eaten type of bone destruction, and associated with an aggressive periosteal response that has an onion skin (or “onion peel”) or, less commonly, a “sunburst” appearance, and a large soft-tissue mass (
Fig. 22.7). Occasionally, the bone lesion itself is almost imperceptible, with the soft-tissue mass being the only prominent radiographic finding (
Fig. 22.8).
On radionuclide bone scan, Ewing sarcoma shows an intense increase of technetium-99m methylene diphosphonate (
99mTc-MDP) uptake. Gallium-67-citrate (
67Ga-citrate) more readily identifies soft-tissue tumor extension. Although scintigraphic findings are nonspecific, this technique provides reliable information concerning the presence of skeletal metastases. CT reveals the pattern of bone destruction, and attenuation values (Hounsfield units) provide information about the medullary extension. In addition, CT may help to delineate extraosseous involvement (see
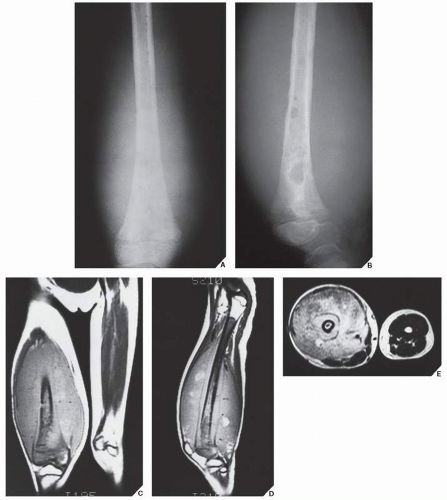
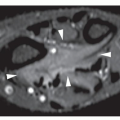
Fig. 22.7). MRI is essential for definite demonstration of the extent of intraosseous and extraosseous involvement by this tumor (
Fig. 22.9). In particular, MRI may effectively reveal extension through the epiphyseal plate. T1-weighted images show intermediate to low signal intensity, which becomes bright on T2 weighting. Hypocellular regions and areas of necrosis are of lesser intensity. Imaging after injection of gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA) reveals signal enhancement of the tumor on T1-weighted sequences. Enhancement occurs only in the cellular areas, allowing differentiation of the tumor from the peritumoral edema.
Histologically, Ewing sarcoma consists of a uniform array of small cells with round hyperchromatic nuclei, scant cytoplasm, and poorly defined cell borders. The mitotic rate is high, and necrosis is frequently extensive. Usually, the cytoplasm contains a moderate amount of glycogen, demonstrable with the periodic acid-Schiff (PAS) stain. This PAS-positive material is washed away after digestion with diastase, confirming that, in fact, it represents glycogen. The demonstration of glycogen, which at one time was considered an absolutely distinctive marker for Ewing sarcoma, has fallen into disfavor because in some Ewing sarcoma, glycogen is not found. Moreover, malignant lymphoma and primitive neural tumors may at times contain glycogen. Since the advent of immunohistochemistry, lymphomas are usually differentiated from Ewing sarcomas by demonstrating leukocyte-common antigen, a pathognomic marker for lymphomas, and primitive neural tumors differ from Ewing sarcomas by the fact that they contain neural protein antibodies. Furthermore, immunohistochemistry reveals that almost all Ewing family tumors exibit a positive membranous and cytoplasmic reaction for CD99 and vimentin, respectively.
Differential Diagnosis
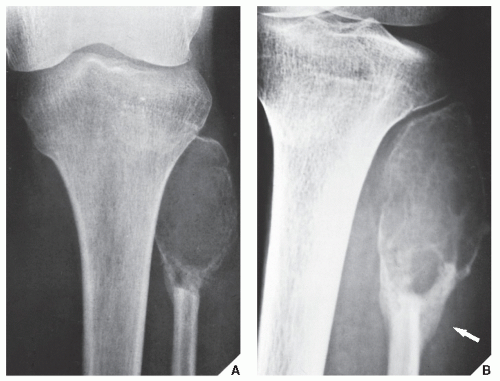
Ewing sarcoma may often mimic metastatic neuroblastoma or osteomyelitis (
Fig. 22.10). At times, Ewing sarcoma exhibits a feature once thought to be almost pathognomonic, the “saucerization” of the cortex (
Fig. 22.11), which may be related to destruction of the periosteal surface by the tumor combined with the effect of extrinsic pressure by the large soft-tissue mass. Although this sign has recently been reported in other tumors, and even in osteomyelitis, its presence in association with a permeative lesion and a soft-tissue mass favors the diagnosis of Ewing sarcoma. The radiographic distinction of Ewing sarcoma from metastatic neuroblastoma may occasionally be difficult; however, the latter usually occurs in the first 3 years, whereas Ewing sarcoma is uncommon in the first 5 years.
Occasionally, Ewing sarcoma may resemble an osteosarcoma, particularly when the former is accompanied by abundant periosteal new bone formation. Moreover, dystrophic calcifications in the soft-tissue mass may mimic tumor-bone formation in osteosarcoma (
Fig. 22.12). Lymphoma must also be included in the differential diagnosis, although
this lesion usually occurs in an older age group. The important radiologic difference is usually the absence of a soft-tissue mass in lymphoma, whereas in Ewing sarcoma a soft-tissue mass is almost invariably present, often being disproportionally large compared with the amount of bone destruction (see
Figs. 22.8 and
22.9). The distinction between Ewing sarcoma and PNET cannot be made on the basis of radiography. Differentiation between these two tumors must rely entirely on immunohistochemistry, electron microscopy, and molecular genetic studies.
Treatment
Ewing sarcoma is usually treated with a preoperative course of chemotherapy, either alone or combined with radiation therapy, to shrink the tumor, followed by wide resection (
Fig. 22.13). Sometimes the affected limb can be reconstructed with an endoprosthesis or an allograft.
Malignant Lymphoma
The term
malignant lymphoma refers to a group of neoplasms that are composed of lymphoid or histiocytic cells of different subtypes in various stages of maturation. Once called “reticulum cell sarcoma,” “non-Hodgkin lymphoma,” “lymphosarcoma,” or “osteolymphoma,” bone lymphoma is now known as
large cell or
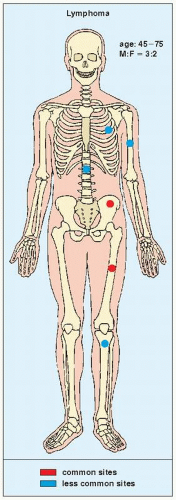
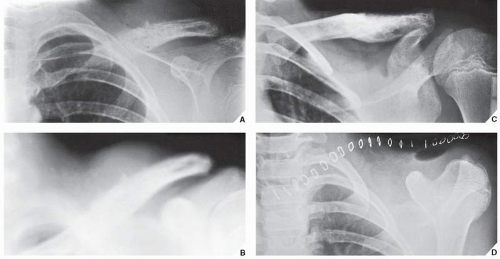
histiocytic lymphoma. According to new WHO classification, malignant lymphomas of bone are subdivided into (a) those that affect one skeletal site with or without involvement of regional lymph nodes; (b) those that affect multiple bones without lymph nodes or visceral involvement; (c) those that present as a primary bone tumor but reveal nodal or visceral lesions; and (d) those occurring in the patients with known lymphoma elsewhere. Groups (a) and (b) are considered primary lymphoma of bone. Primary bone lymphoma is a rare tumor that accounts for less than 5% of all primary bone tumors. It occurs in the second to seventh decades, with a peak age of occurrence from 45 to 75 years; it has a slightly greater prevalence in males. The lesion develops in the long bones, vertebrae, pelvis, and ribs (
Fig. 22.14). Patients may present with local symptoms, such as pain and swelling, or with systemic symptoms, such as fever and weight loss.
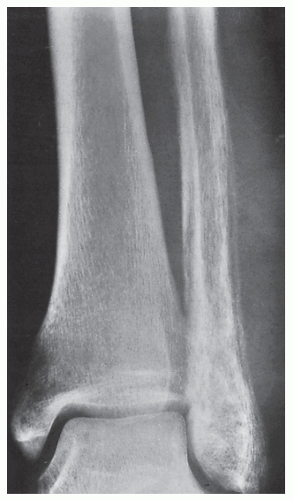
Radiographically, histiocytic lymphoma produces a permeative or moth-eaten pattern of bone destruction or is a purely osteolytic lesion with or more commonly without a periosteal reaction (
Fig. 22.15). The affected bone can also present with an “ivory” appearance, as is often the case in lesions of the vertebrae or flat bones. Pathologic fractures are occasionally encountered (
Fig. 22.16, see also
Fig. 22.18). Because lymphoma usually does not evoke significant periosteal new bone formation, this is an important feature in differentiating it from Ewing sarcoma.
Recently, WHO adopted the Revised European-American Classification of Lymphoid Neoplasms (REAL) that originally was proposed by the International Lymphoma Study Group (
Table 22.1).
Histologically, lymphomas may be subdivided into non-Hodgkin lymphomas and Hodgkin lymphomas. Although secondary involvement of bones is relatively common in Hodgkin lymphoma, primary Hodgkin bone lymphoma is extremely rare. Non-Hodgkin bone lymphomas are considered primary only if a complete systemic workup reveals no evidence of extraosseous involvement. Histologically, the tumor consists of aggregates of malignant lymphoid cells replacing marrow spaces and osseous trabeculae. The cells contain irregular or even cleaved nuclei. As mentioned in the section on Ewing sarcoma, the most important single procedure used to distinguish lymphoma from the other round cell tumors is the stain for leukocyte-common antigen, because lymphoid cells are the only cells that stain positively with the immunoreaction for CD45, CD20, and CD3 (B-cell and T-cells markers).
Differential Diagnosis
Histiocytic lymphoma must be distinguished from secondary involvement of the skeleton by systemic lymphoma. It may resemble Ewing sarcoma, particularly in younger patients (
Fig. 22.17), or Paget disease if the articular end of a bone is involved and there is a mixed sclerotic and osteolytic pattern (
Fig. 22.18).
Treatment
The treatment for primary bone lymphoma is controversial, and there is no consensus with regard to radiotherapy, although this tumor is radiosensitive. Some cases require chemotherapy as the mainstay and additional adjuvant radiation therapy. The optimal treatment has not been determined and is still being debated.
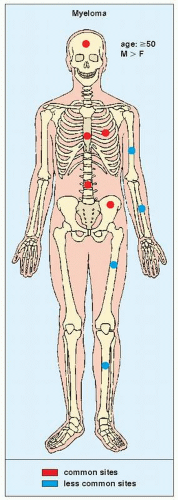
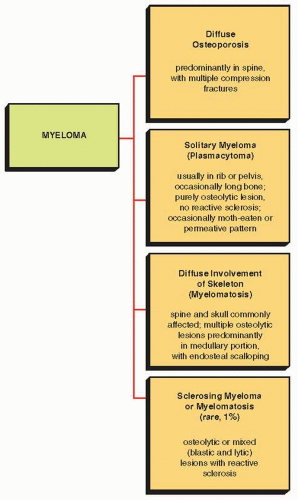
Myeloma
Myeloma, also known as “multiple myeloma” or “plasma cell myeloma,” is a tumor originating in the bone marrow and is the most common primary malignant bone tumor. It accounts for 10% of all hematological malignancies and 1% of all cancers. It is usually seen between the fifth and seventh decades and is more frequent in men than in women. The axial skeleton (skull, spine, ribs, and pelvis) are the most commonly affected sites, but no bone is exempt from involvement (
Fig. 22.19). Rarely, the presentation can be that of a solitary lesion, in which case it is called a
solitary myeloma or
plasmacytoma; far more commonly, however, it presents with widespread involvement, in which case the name
multiple myeloma is applied. Mild and transient pain exacerbated by heavy lifting or other activity is present in approximately 75% of cases and may be the initial symptom. Because of this, in its early course and before diagnosis, the disease may resemble sciatica or intercostal neuralgia. Rarely, a pathologic fracture through the lesion is the first sign of disease. The patient’s urine in cases of myeloma contains Bence Jones protein; the serum albumin-to-globulin ratio is reversed and the total serum protein is elevated. Monoclonal γ-globulin is also present, with IgG and IgA peaks demonstrated on serum electrophoresis.
Histologically, the diagnosis is made by finding sheets of atypical plasmacytoid cells replacing the normal marrow spaces. The plasma cell is recognized by the presence of eccentrically situated nucleus within a large amount of cytoplasm that stains either light blue or pink. The neoplastic cells contain double or even multiple nuclei, usually hyperchromatic and enlarged, with prominent nucleoli.
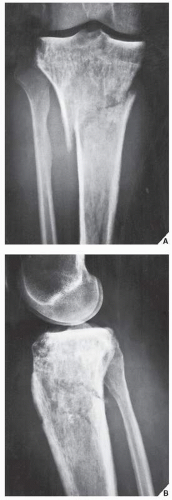
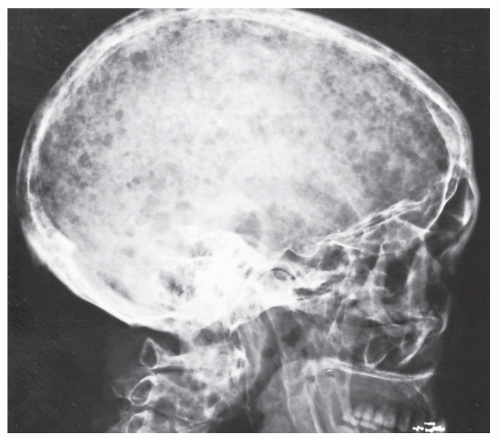
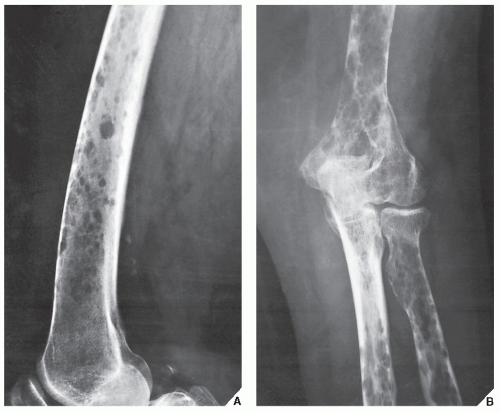
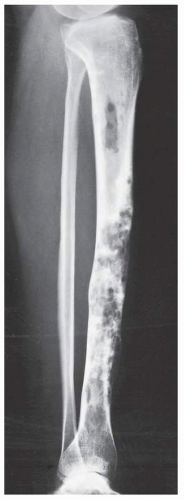
Multiple myeloma may present in a variety of radiographic patterns (
Fig. 22.20). Particularly in the spine, it may be seen only as diffuse osteoporosis with no clearly identifiable lesion; multiple compression
fractures of the vertebral bodies may also be evident. More commonly, it exhibits multiple lytic lesions scattered throughout the skeleton. In the skull, characteristic “punched-out” areas of bone destruction, usually of uniform size, are noted (
Fig. 22.21), whereas the ribs may contain lace-like areas of bone destruction and small osteolytic lesions, sometimes accompanied by adjacent soft-tissue masses. Areas of medullary bone destruction are noted in the flat and long bones, and if these appear about the cortex, they are accompanied by scalloping of the inner cortical margin (
Fig. 22.22). Ordinarily, there is no evidence of sclerosis and no periosteal reaction. Fewer than 1% of myelomas may be of a sclerosing type called
sclerosing myelomatosis.Whereas in osteolytic myeloma only 3% of patients have polyneuropathy, the incidence of polyneuropathy in the osteosclerotic variant has been reported as 30% to 50%. Compared with classic myeloma, this variant usually occurs in younger individuals and shows fewer plasma cells in the bone marrow, lower levels of monoclonal protein, and a better prognosis.
An interesting variant of sclerosing myeloma is the so-called POEMS syndrome, first described in 1968 but gaining wide acceptance only more recently. It consists of polyneuropathy (P), organomegaly (O), particularly of the liver and the spleen, endocrine disturbances (E) such as amenorrhea and gynecomastia, monoclonal gammopathy (M), and skin changes (S) such as hyperpigmentation and hirsutism. Also known as Crow-Fukase syndrome, Takatsuki syndrome, and PEP (plasma cell dyscrasia, endocrinopathy, and polyneuropathy) syndrome, this condition represents a clinicopathologic complex of unknown etiology. On radiography and CT, the focal osseous lesions present as either a well-defined or fluffy sclerotic foci, or as a lytic areas with peripheral sclerosis. On MRI, the lesions exhibit decreased signal intensity on both T1- and T2-weighted sequences, and lack of enhancement on postcontrast (gadolinium) images.
Differential Diagnosis
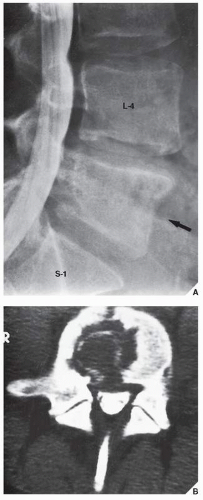
If the spine is involved, as is frequently the case, multiple myeloma must be differentiated from metastatic carcinoma. In this respect, the “vertebral pedicle” sign identified by Jacobson and colleagues may be helpful. They contended that in the early stages of myeloma, the pedicle (which does not contain as much red marrow as the vertebral body) is not involved, whereas even in an early stage of metastatic cancer the pedicle and vertebral body are both affected (
Fig. 22.23). In the late stages of multiple
myeloma, however, both the pedicle and vertebral body may be destroyed. Radionuclide bone scan can more reliably distinguish these two malignancies at this stage. It is invariably positive in cases of metastatic carcinoma, whereas in most cases of multiple myeloma there is no increased uptake of radiopharmaceutical tracer. This phenomenon appears to reflect the purely lytic nature of most myelomatous lesions and the absence of significant reactive new bone formation in response to the tumor.
A solitary myeloma may create even greater diagnostic difficulty. As a purely osteolytic lesion, it may mimic such other purely destructive processes as the brown tumor of hyperparathyroidism, giant cell tumor, fibrosarcoma, MFH, or a solitary metastatic focus of carcinoma from the kidney, thyroid, gastrointestinal tract, or lung.
Complications and Treatment
A common complication of bone myelomas is pathologic fracture, especially in lesions of the long bones, ribs, sternum, and vertebrae. The development of amyloidosis has also been reported in approximately 15% of patients.
Treatment consists of radiotherapy and systemic chemotherapy. The 5-year survival rate is approximately 10%.
Adamantinoma
Adamantinoma is a rare malignant tumor occurring equally in males and females between the second and fifth decades of life; 90% of cases involve the tibia. Radiographically, the disease is marked by well-defined and elongated osteolytic defects of varying size, separated by areas of sclerotic bone, which occasionally give the lesion a “soap bubble” appearance; ordinarily, there is no periosteal reaction (
Fig. 22.24). At times, adamantinoma may affect an entire bone with multiple satellite lesions (
Fig. 22.25); “sawtooth” areas of cortical destruction in the tibia are quite distinctive of this tumor.
Histologically, the tumor is biphasic and consists of an epithelial component intimately admixed in varying proportions with a fibrous component. Although it has been speculated that adamantinoma represents a form of vascular neoplasm, ultrastructural and immunohistochemical evidence points toward an epithelial derivation.
A relationship of adamantinoma with osteofibrous dysplasia and fibrous dysplasia has been postulated and its coexistence with either of these lesions has been suggested. However, this is still controversial, with some investigators maintaining that the lesions of adamantinoma may contain a fibro-osseous component that can resemble a Kempson-Campanacci lesion or fibrous dysplasia on histopathologic examination. (See also discussion in
Chapter 19 in the section on Osteofibrous Dysplasia.)
Treatment
Because adamantinoma is insensitive to radiotherapy, the treatment of choice is en bloc surgical resection with application of bone graft. Recurrences have been reported.