MRI equipment
Picker 1.5 edgepicker 1.5 edge
Siemens Espree 1.5 T
GE 3 T signa HDxt
Philips Achieva 1.5 T
Sequences
FSE
Haste
ss-FSE3
ssh-TSE4
TR
3500
850
2399
712
TE
168
101
141
110
FOV
290–360
300–400
300–400
300–400
Thickness
8
6
6
6
Matrix
192 × 256
320 × 320
320 × 320
288 × 288
During the second stage, obstetricians requested for pMRI to know the degree of invasion [10], although this difference usually didn’t modify the tactic or surgical technique. In addition, it is known that different degrees of invasion usually coexist in the same patient [11]. This difference was recognised by a pathologist, who says that a simple sample is not a gold standard for diagnosis of abnormal invasive placentation [12].
During the third stage, pMRI was requested to know the anatomy of invasion, especially to examine the parametrium and the interface among placenta, invaded myometrium and the bladder [9–13]. But for a few authors, this information didn’t improve or modify the surgical plan. Part of this is a consequence of using long-standing techniques (1933) such as to leave the placenta in situ [14], but mainly because obstetricians are not formally trained to read these images. This situation also happens with radiologists, who perform the study without previous formal training in AIP images [15]. The lack of background after the surgery to re-evaluate the image findings is part of this problem. At a point, pMRI has a big difference compared with ultrasound, because US is performed mainly by obstetricians who have access of to perform the surgery, a situation that allows building a background of informed images immediately.
However, pMRI is an excellent tool for abnormal invasive placenta evaluation, because it is a multiplanar study with total acquisition of the affected area [16]. This fact makes it possible to study specific zones in detail not easily accessible with ultrasound, like the parametrium or posterior invasions. Besides, and as it was mentioned before, different degrees of invasion may coexist in the same patient, and although it is not common, the same patient may have an anterior placenta accreta and a massive parametrial invasion, information that completely modifies the surgical technique [17].
Due to the potential life threat of this condition, the use of different image techniques increases confidence before starting the surgery. In other words, ultrasound, Doppler and pMRI are not different methods to confirm the same diagnosis; they are complementary techniques to obtain different information to make active decisions in order to plan and anticipate further complications [18].
14.2 Definitions and Diagnostic Problems
For a long time, obstetricians and image studies used a histological classification of placental invasion to inform images of AIP cases, but these criteria may end in surgical problems or misinterpretations [11]. Histological classification names as placenta accreta are those placentas that invaded the myometrium without interposition of tunica basalis. Those placentas that invade the myometrium deeply are called placenta increta, and the term percreta is for those in which placental invasion reaches the myometrium or passes beyond it. But, due to the fact that most abnormal placentations are located in or near the previous scar, most of them should be called percretas, because the scar area is generally thin and the placenta usually reaches the serosa, a fact that from a surgical point of view is not right. So, the use of a histological classification can be confusing to perform a prenatal diagnosis of AIP. Likewise, when the placenta protrudes by a dehiscent scar, this would be termed percretas from histological point of view, a fact that is incorrect and it may be the cause of definitive over treatment like hysterectomy, even in young women [19]. For this reason, we need to know that diagnosis of AIP has some limitations which may confirm and also possibly modify the prenatal plan after surgical exploration.
From a prenatal and surgical point of view, it is possible to classify these placental invasions into 3 groups:
1.
AIP grade 0 (false AIP): In this case, the placenta protrudes through the scar, the myometrium is too thin, but there is no evidence of newly formed vessels or other problems in the uterine-bladder interface (Fig. 14.1). Images may show an apparent placental tissue, which reaches or seems to be inside the bladder. They are cases with scarce signs during US evaluation, while pMRI allows discarding a possible invasion by multiplanar analysis. In these cases, when the obstetrician decides to leave the placenta in situ, it is common for it to be expelled in a short time without bleeding or other complications.
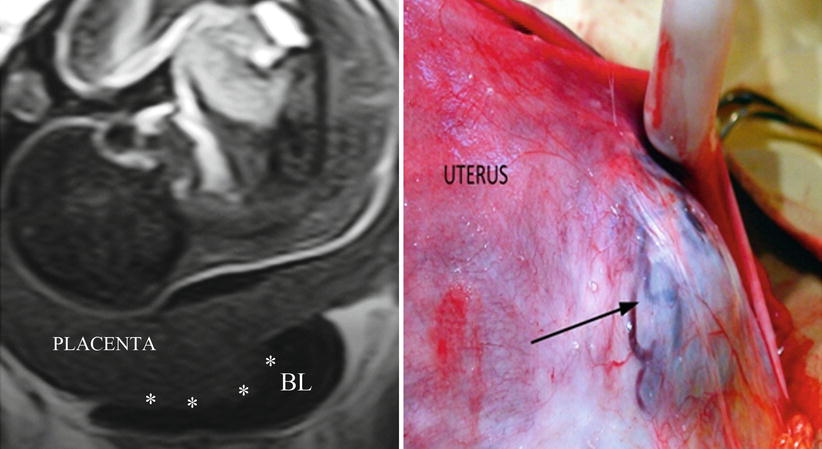

Fig. 14.1
Left: coronal T2 image, the placenta protrudes and enters in the bladder (white asterisks). There are not newly formed vessels visible in this area. Right: surgical view, the placenta bulges through a previous hysterotomy. There are no vascular connections between uterus and the bladder. First ultrasound was informed by placenta percreta, but second specialised one modifies this diagnosis by scar dehiscence or AIP grade 0 (uterine scar dehiscence)
2.
AIP grade 1 (classical AIP): In this group, the placenta has specific modifications that agree with the growth factor stimulation, such as lacunae, lobulations and dark bands. It is possible to see a blurred uterine-bladder interface, the uterine segment may be interrupted in small sectors, and there are scarce vessels between placenta and bladder (Fig. 14.2). This group agrees with the histological classification of placenta accreta or increta.
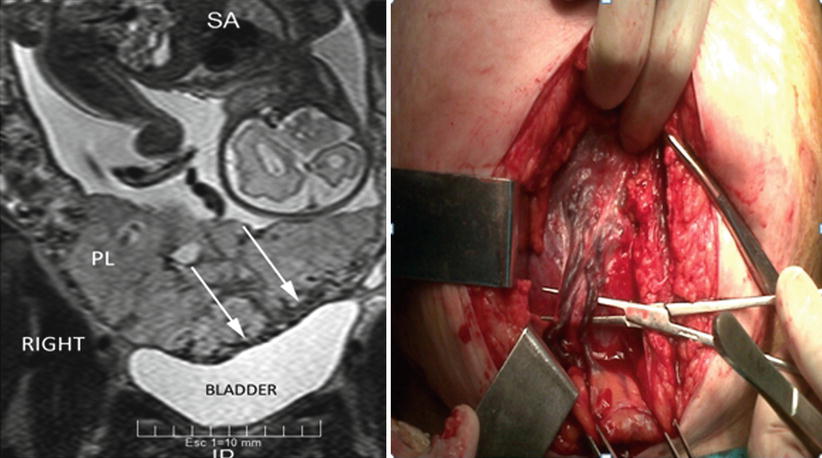

Fig. 14.2
Left: coronal T2 image, the placenta invades an external layer of the bladder. Circular hypointensive signals (newly formed vessels) are clearly seen in the uterine-vesical interphase (white arrows). The myometrial signal is absent in most part of invaded area. Right: surgical view, presence of newly formed vessels was evident after laparotomy from the placenta-myometrium to the bladder
3.
AIP grade 2 (highly invaded AIP): In these cases, anatomical evidence of invasion is high, especially with the presence of thick or multiple newly formed vessels among placenta, invaded myometrium and the detrusor (bladder muscle). The myometrium is highly invaded especially in the uterine segment, with a particular tendency to rupture and bleed during dissection (Fig. 14.3). Viewing of placental lagoons, newly formed vessels and placental sacculations is evident. When the relation between placenta, invaded myometrium and the bladder is really stuck, it is called as bladder invasion. But under a strict point of view, this is not a real organ invasion like a malignancy; it is a vascular invasion from placental-myometrial vessels to the detrusor muscle, all involved inside different degrees of adherent tissue process [11].
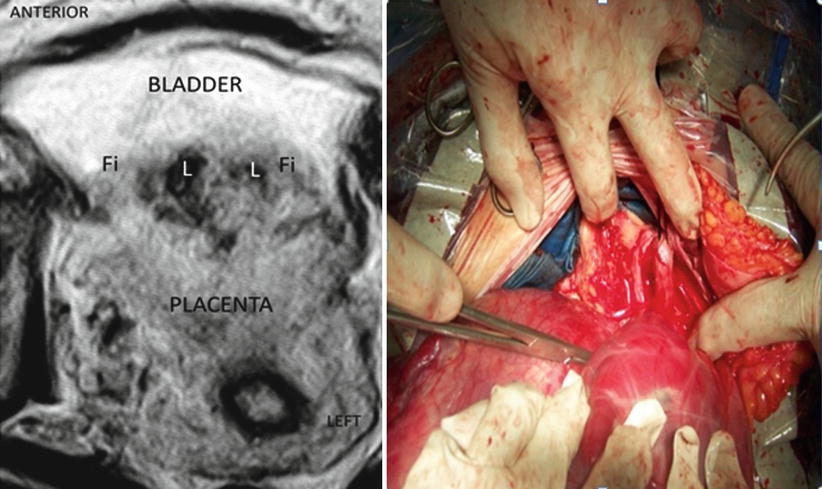

Fig. 14.3
Left: axial T2 image: posterior bladder wall is invaded by placenta. There is no identifiable plane between uterus and the bladder. The presence of irregular lagoons is compatible with AIP. Right: surgical view, total invasion of posterior wall of the bladder. Tissues between organs are really stick and also adherent to the right parametrium. These tissues are prone to the rupture and bleeding during dissection
AIP grade 0, 1 and 2 may also extend to the lateral part of the uterine segment (parametrium), although the most common is the grade 0 or 1. Grade 2 is particularly unusual but common in initial uterine segment lateral implantations (Fig. 14.4). The presence of vessels associated to this type of lateral invasions is evident in axial pMRI slices. This surgery is usually a nightmare because access to this deep and narrow area is quite difficult due to the presence of communication vessels from the ureter and pelvic wall branches from the iliac internal and even from the iliac external artery [11].
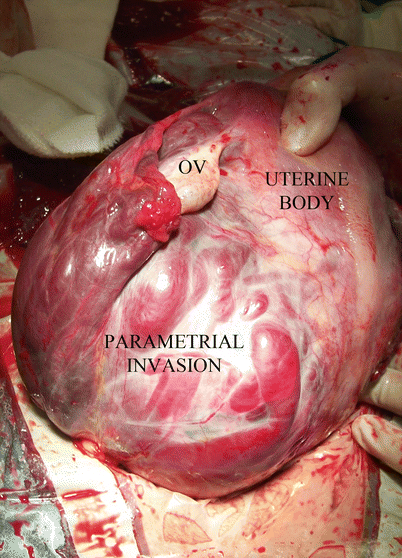

Fig. 14.4
Surgical view: complete and left lateral parametrial invasion. All lateral side of the uterus is infiltrated by the placenta. Patient background: 1 CS, curettage after caesarean by aberrant low cotyledon, second pregnancy after 4 months of previous CS
This disparity between histology, images and surgical features can be confusing to make decisions, especially for those obstetricians not specialised in this condition (Fig. 14.5). For this reason, agreement on diagnosis and terms is highly recommended to know exactly what type of invasion is described and then which was a better treatment for them. Many centres discuss all the prenatal and postnatal findings among all specialists involved, who comment on weaknesses and strengths in diagnosis in order to learn and modify surgical approaches and techniques. Although this learning activity is enriching in terms of improving diagnosis and treatments, for many reasons, it is not a wide-ranging practice.
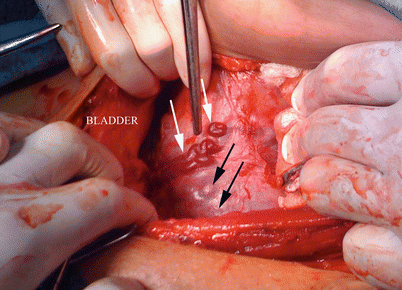

Fig. 14.5
Surgical view: parallel vessels over uterine segment surface (white arrows). Peripheral placental circulation (black arrows) plus parallel vessels could mimic placenta percreta. Surgical exploration in patients with AIP is necessary to confirm prenatal diagnosis and avoid under or over treatment
14.3 Obstetric MRI Versus pMRI
Although at first both studies, obstetric MRI and pMRI, may be considered the same, analysis of the placenta needs a special attention in order to get significant and useful images for diagnosis and surgical planning. Obstetrical MRI is indicated to study a wide range of foetal anatomy, such as spine, neck, nervous system, thorax, urogenital tract and extremities, among others. Placental MRI places a special focus on the placental anatomy in relation to the surrounding tissues, especially with the bladder and pelvic organs. The information provided by pMRI is particularly important as a prenatal guide to perform a surgical approach and also the vascular control. The image must include all the pelvic anatomy very clearly and not in the peripheral area as an obstetric MRI.
14.4 Placental MRI: Diagnosis
During the last decade, there were a great number of studies that analysed and compared the sensitivity and specificity of pMRI with ultrasound and Doppler [20]. None of them showed significant differences, although availability and cost favour ultrasound. It is common that after initial work by Dr. D. Levine [21], most authors accepted some pMRI advantage for the posterior invasions in comparison to US diagnosis [22]. However, none of these studies showed any image of posterior invasions. Analysis of ultrasound diagnostic failure in AIP cases is not demonstrative of specific causes. It is usual to read in papers that ultrasound was not able to perform a diagnosis, but there is no analysis regarding why. It is probable that this lack of prenatal diagnosis is associated to expertise and interpretation of US signs [6]. Recently, some authors have cast some doubt on the values of classic ultrasound signs, to indicate a lower value than what was published before. But there are no formal experiences to evaluate the same US study among obstetricians, although recent international experiences have shown a wide range of opinions and diagnosis among AIP experts and nonspecialists. These differences were minor or inexistent among AIP specialists, a fact that suggests two possible levels of competence: one that can be made by nonspecialists, who can determine cases which qualify as highly suspicious, and other levels that can be made by specialists in AIP diagnosis, who are able to provide accurate information to make definitive decisions [13].
Initially it was believed that pMRI improved or corrected these diagnostic differences, but as it happens with ultrasound or with Doppler, pMRI is a study that needs a deep experience and continuous feedback [11]. Some details about AIP are relevant to know why study preparation is important. Among positive or significant signs of MRI signs, like presence of lagoons, thinning of myometrium (Fig. 14.6) the existence of newly-formed vessels is one of the most important to diagnose AIP. These vessels are a consequence of the action of growth factors over the microanastomotic vessels between the uterus and the surrounding tissues, usually the bladder. These vessels are not strictly newly formed, because they are connected organs although are unnoticed to the naked eye. Due to the fact that they are not under normal arterial pressure, the tunica media (muscular) is poorly developed or practically inexistent [11]. For this reason, these newly formed vessels collapse very easily. To be evident in the pMRI, they mustn’t compressed by the bladder. For this reason, the bladder could be semi-filled at the time of performing a pMRI, because it is the best way to see the abnormal circulation. If the bladder is empty, the placenta crushes vessels and the bladder walls against the symphysis, and they are not seen in the study. On the other hand, if the bladder is overdistended, the newly formed vessels are collapsed against the placenta, and also, they are not visible by pMRI [9]. A better visualisation of the uterine-vesical interface could be done if the patient intake 600 ml of liquid 45 min before the study.
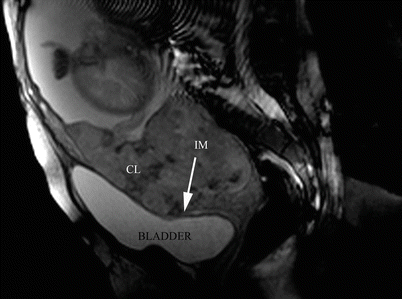

Fig. 14.6
Sagittal T2 image: confluent lagoons (CL) are evident near to uterine-bladder interphase. The uterine segment myometrium is interrupted (IM) or extremely thinning. In this area, the placenta reaches to the uterine serosa
Anterior AIP is the most common location worldwide, because it is also the most common site of the previous scar (caesarean). However, the lateral part of the uterus (parametrium) could not be seen properly by US due to the lack of natural contrast or liquid. But in recent years, some studies have investigated the possibility to explore the parametrial area in cervical cancer [23, 24]. Although parametrial invasions are not common in all countries, it is a condition relatively frequent associated after unsafe abortions. This variety must also be suspected in cases of lateral retained placenta, curettage by aberrant cotyledon or by curettage after short-time caesarean interval [11]. Coronal and axial slices of pMRI are really accurate (Figs. 14.7 and 14.8) to demonstrate this kind of invasion [13]. As it was commented before, lateral invasions may have two subtypes: (1) There is a lack of support of myometrium, because it appears thin. The placenta clearly protrudes until the lateral pelvic wall. (2) Besides placenta protruding (lateral bulging) a vascular signal of engorged vessels is evident around the invasion. The last type of invasion is very rare, though highly problematic to solve. Although other invasions are suitable to leave the placenta in situ, this is particularly dangerous in parametrial invasion type 2. Postpartum uterine contractions may produce an unexpected laceration of the weak lateral myometrium and start a massive haemorrhage. An emergency scenario includes a patient with a severe hypovolaemic shock, intra-abdominal or retroperitoneal bleeding and invaded placenta in a deep space plenty of enlarged vessels, which is a true surgical nightmare [25]. Except for a few special cases, this episode is the cause of death, because it is almost impossible to solve all problems very quickly. Although these cases are not reported or published, the specialist knows about them through informal talking with other specialists in congresses.
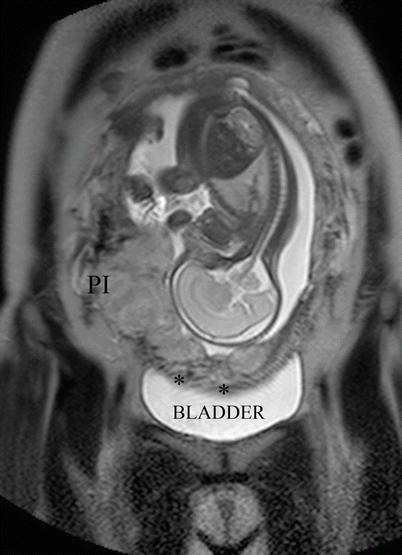
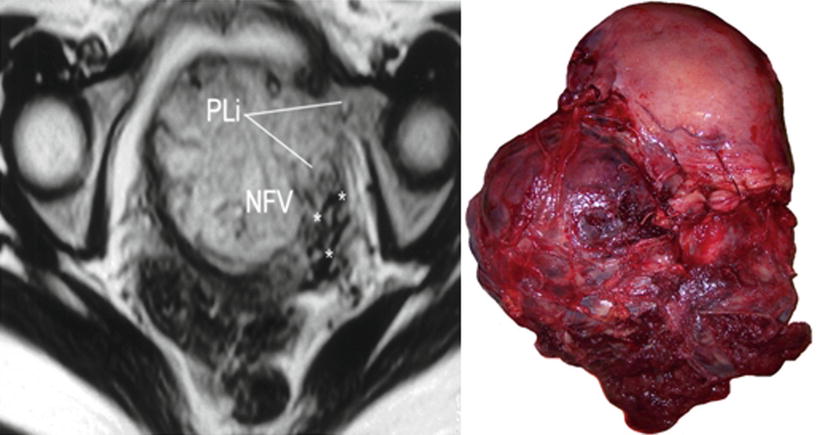

Fig. 14.7
Coronal T2 image: MRI was requested by US doubt of anterior invasion (black asterisk). Parametrial invasion (PI) was evident in the right side of the uterus. After study, the patient admitted having an abortion

Fig. 14.8
Left: axial T2 image, PLi parametrial invasion, NFV newly formed vessels (asterisks). Right: massive low parametrial invasion. Uterine segment was not invaded by the placenta. MRI was requested by US doubt of anterior invasion
Although the number of cases with parametrial invasion is not known, diagnosis of this variation could imply several changes in the surgical plan [13]. Parametrial types 1 and 2 are formal indications of ureteral catheterisation in order to prevent unexpected damage during resective procedures (hysterectomy). When catheterisation is not possible, surgical identification is extremely useful to prevent ureteral damage. Although this manoeuvre is usually performed by urologist, it is sometimes very difficult because of the lack of anatomical landmarks, which are modified by placental protruding against a lateral pelvic wall [26]. Diagnosis of parametrial invasion type 2 suggests a possibility of heavy bleeding from vessels that have no relation with the uterine artery. In these cases, an accurate and efficient method of proximal vascular control must be performed before starting a hysterectomy. In the low parametrial area, the vessels come from different sources, and embolisation is usually not useful at all (Fig. 14.9). Attempt to control in these cases implies a high risk of failure and also a nontarget embolisation.
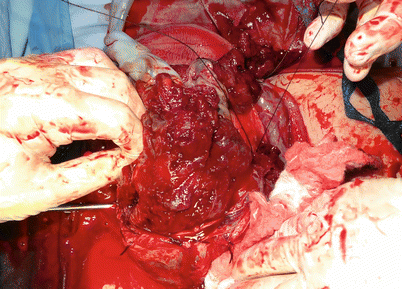

Fig. 14.9
Surgical view: unexpected parametrial invasion discovered during hysterectomy. Iliac internal occlusion was not effective to stop the bleeding and dissection was unmanageable. Emergency aortic balloon was inserted and an expertise group was called to complete the surgery
14.5 Placental MRI: Topography of the Invasion
Historically, 2 pedicles have been described for uterine irrigation, the uterine and the ovary arteries. However, after the use of embolisation to manage different types of obstetric haemorrhage, some complications and failures couldn’t be explained according the classic anatomy. A preliminary study made to use a uterine compression device (myomas treatment) demonstrated that lateral compression of uterine tissues above uterine arteries produces uterine necrosis in 6 h [27]. This was the first published study that demonstrated that the upper pedicle (ovary and round ligament artery) is not able to replace the uterine blood flow after uterine artery ligature or embolisation. A few years later, an anatomical study showed a thick and wide communicated anastomosis between vaginal and uterine arteries [28]. Irrigation areas from upper and lower uterine pedicles are different and also their origin. Uterine arteries arise from the anterior division of iliac internal artery, while vaginal ones arise from the posterior division. A practical division of uterine irrigation areas is the peritoneal reflection, which determines area S1 (above peritoneal reflection) and area S2 below this. S1 sector is mainly irrigated by the uterine arteries, while S2 sector by collaterals of the pudendal internal arteries. The topography of placental invasion indicated which is the most effective method for proximal vascular control [29]. For S1 invasions (less frequent in AIP), anterior iliac internal control is effective, but for S2 placental invasions, it is necessary to control the blood flow of the pudendal internal branches and their anastomotic connection, so the most accurate vascular control is the iliac common or aortic vascular control [30].
Identification of S1 and S2 areas can be established by pMRI, sagittal slice (Fig. 14.10). A line that perpendicularly crosses the middle of the posterior bladder wall determines an upper area named S1, which mainly corresponds to the uterine body, and an area below this line, named S2, which involves the lower segment, cervix and upper vagina [9]. Most part of AIP is located in S2 area, which also explained the high rate of failures with the use of uterine or internal iliac vascular control.
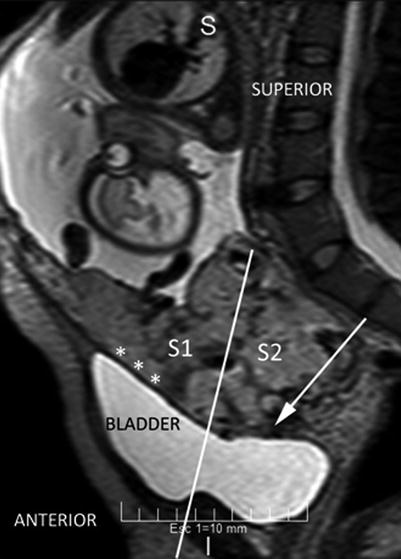

Fig. 14.10
Sagittal MRI T2 image: perpendicular plane which divide the posterior bladder wall determine an areas S1 and S2. White arrow shows an interrupted myometrium. Lagoons and vessels are clearly visible in the myometrial-bladder interphase
14.6 Placental MRI: Depth of Invasion
During a second stage, pMRI was requested to know the depth of placental invasion [10], especially to know whether the bladder is invaded. But bladder invasion is a vascular phenomenon, completely different from tissue invasion than neoplasia. In these cases, the placenta takes irrigation from the external layer of the bladder, but the state of uterine-bladder interface may be different according to thinning of myometrium, bladder wall, fibrous tissue between them or a combination of all these variables. For some authors, when the bladder is compromised, this is a formal indication to leave the placenta in situ, but this is not a really mandatory option. Placental MRI allows seeing this interface in great detail in axial T2 slices. When this series (axial T2) is performed perpendicularly to the posterior bladder wall, the anatomy of the placental-bladder interface is not distorted and shows a clear anatomy [13]. This interface can be distorted by peripheral circulation in a placental surface (engorged superficial vessels and lagoons) or by the presence of newly formed vessels between the placenta and the bladder. The presence of circular hypointensive signals (newly formed vessels) in the lower and posterior part of the bladder (cervicotrigonal area) is a reasonable sign of a very difficult dissection [13].
Placental MRI is a total acquisition study; for this reason, it allows analysing the whole invaded area, which can show different invasion degrees in the same patient. Sagittal slices are more suitable to evaluate the integrity and thickness of the anterior uterine wall in perspective. This is especially helpful when conservative-resective technique is the plan, because it allows knowing if there is healthy myometrium below the invaded area to perform a uterine anterior wall repair (prior to the surgery). As it was mentioned before, parametrial invasion is not a common event, but when it’s present, the surgery could change radically. For this reason, it is important to highlight that pMRI is not an initial method to perform diagnosis of AIP, but some additional information, especially topographic, like parametrium [9–22], may not be available with other methods. Although some specialists consider that the cost of MRI does not justify its use in AIP, when serious complications appear [31–35], it is obvious that the cost is completely validated to reduce severe complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree