Miscellaneous Uses of Cardiovascular Magnetic Resonance Imaging
Robert W. W. Biederman
Perhaps the most interesting aspect of cardiovascular magnetic resonance (CMR) involves the myriad number of novel applications to which it can be applied. Given the multitude of CMR sequences, combined with the plasticity with which they can be utilized, one might feel that we are only limited by our imagination. Innovative uses for CMR can be obtained by using standard sequences, but applied in a nonstandard manner. This aspect is distinctly different as compared to other modalities that have limited body applications, as well as limited physical characteristics, which prevent their widespread application. It is for this reason that great creativity can be brought to bear by the imaging physician and technologists. Listed in the following text are a few of the more interesting and imaginative application areas.
CONSTRICTIVE PERICARDITIS
Evaluation of constrictive pericarditis in conventional modalities requires high-quality imaging of the pericardium. Echocardiography has a well-defined limited ability to delineate the anatomic features of the pericardium. Although the posterior wall is often seen to be thick, it is due to a far-field artifact, generating a hyperechoic signal, often mimicking a thickened, highly calcified pericardium. Pulse-wave Doppler techniques demonstrating evidence of a restrictive pattern, as well as an abnormal tissue Doppler signal with an exaggerated “e” and blunted “a” wave are helpful in the evaluating the presence of constricted physiology. When a respirometer is available, the perturbed physiology can be evaluated more accurately, whereupon inspiration and expiration produce a characteristic Doppler pattern that can aid in diagnosis. In the catheterization suite, reliance on a morphologic “square root sign” is the cardinal feature. When fluid boluses are given, the specificity is increased, but still is far from diagnostic in many cases. A technique that has received some popularity in the catheterization suite is using the concept of “ventricular interdependence” in which during inspiration and expiration there is a characteristic separation in contraction of right and left atrial pressures. This can be a cumbersome technique, and requires invasive instrumentation to make this diagnosis, but in expert hands has high accuracy. Because physical examination, although helpful, has very limited sensitivity and specificity and is chiefly detected by a most subtle pericardial knock, is often not present until middle stages of disease, requiring an astute clinician and high clinical suspicion, there exists a great need for a technique that is both noninvasive, easy-to-use, and near foolproof. It is important to note that the ability to accurately detect constricted pericarditis has tremendous upside and downside potential (see Fig. 16-1). In these patients, who often present late, with months to years of vague progressive indolent symptoms that culminate in severe right heart failure, the accurate diagnosis leads to median sternotomy and pericardial stripping with dramatic improvement in the patient’s symptomatology (see Fig. 16-2). However, in the same setting, with the patient in extremis, an incorrect diagnosis can lead to needless open-heart surgery. Consider the following hypothetical conversation with the cardiothoracic surgeon, in whom you have misdiagnosed constriction in a patient, “Would you please come down to the operating room to show me where you think I should cut the pericardium. By the way, would you mind telling the patient why I needlessly opened this patient up?” When stated in this graphic manner, it is clear that a versatile technique, such as CMR, would be advantageous (see Fig. 16-3).
The technique of radiofrequency tissue tagging, in which saturated lines of magnetization are placed in
either a radial or gridlike pattern across the myocardium noninvasively, is useful in assessing pericardial disease. Typically, radiofrequency tagging is used to determine myocardial contraction patterns, and has been well described by us and others to provide knowledge of segmental myocardial deformation and measurement of strain in a transmural manner across the myocardium. This has been used to evaluate the post-myocardial infarction (MI) remodeling process and assess the effects of myocardial performance before and after surgical intervention. Measurement of circumferential, longitudinal, and radial strain patterns in one, two, and three dimensions has been performed, detailing high-fidelity physiologic patterns of normal and deranged
physiology. However, the clinical applicability is only now becoming recognized for quantitation of myocardial mechanics using this technique. An offshoot of this technology has been applied to evaluation of constrictive pericarditis in which tissue tagging has proved to be a robust and clinically applicable technique. The grid pattern is placed over the entire field of view (FOV), which includes the myocardium and pericardium by definition, and allows separation of pericardial signal from myocardial signal. Except for bulk translation, the pericardial signal is fixed in healthy individuals; therefore the epicardial signal should “slide” past the pericardial surface during systolic contraction and diastolic relaxation. Therefore, the visceral surface of the pericardium (epicardium) should migrate past the stationary parietal pericardium. Failure to see slippage between the visceral and parietal pericardium, evidenced by lack of relative motion, is diagnostic of adherence between the two layers. The observation of a fixed or grid pattern, without slippage, demonstrates that there is fibrosis, scarring, or union between the parietal and visceral pleura. This observation is the sine qua non of constrictive pericarditis (see Fig. 16-4). In our practice, in which we see sliding between the two pericardial layers, despite evidence by echocardiography or catheterization suite of constriction, we are confident in assuring the surgeon that there is no reason to take the patient to surgery. On the other hand, despite absence of constriction by other imaging modalities including the presence of a thin pericardium on computed tomography (CT), if there is no slippage in the major portion of the pericardium by CMR, we can assure the surgeon
that they will find an adherence pattern consistent with constrictive pericarditis upon opening the chest. In our series of more than 50 patients, we have never been incorrect.
either a radial or gridlike pattern across the myocardium noninvasively, is useful in assessing pericardial disease. Typically, radiofrequency tagging is used to determine myocardial contraction patterns, and has been well described by us and others to provide knowledge of segmental myocardial deformation and measurement of strain in a transmural manner across the myocardium. This has been used to evaluate the post-myocardial infarction (MI) remodeling process and assess the effects of myocardial performance before and after surgical intervention. Measurement of circumferential, longitudinal, and radial strain patterns in one, two, and three dimensions has been performed, detailing high-fidelity physiologic patterns of normal and deranged
physiology. However, the clinical applicability is only now becoming recognized for quantitation of myocardial mechanics using this technique. An offshoot of this technology has been applied to evaluation of constrictive pericarditis in which tissue tagging has proved to be a robust and clinically applicable technique. The grid pattern is placed over the entire field of view (FOV), which includes the myocardium and pericardium by definition, and allows separation of pericardial signal from myocardial signal. Except for bulk translation, the pericardial signal is fixed in healthy individuals; therefore the epicardial signal should “slide” past the pericardial surface during systolic contraction and diastolic relaxation. Therefore, the visceral surface of the pericardium (epicardium) should migrate past the stationary parietal pericardium. Failure to see slippage between the visceral and parietal pericardium, evidenced by lack of relative motion, is diagnostic of adherence between the two layers. The observation of a fixed or grid pattern, without slippage, demonstrates that there is fibrosis, scarring, or union between the parietal and visceral pleura. This observation is the sine qua non of constrictive pericarditis (see Fig. 16-4). In our practice, in which we see sliding between the two pericardial layers, despite evidence by echocardiography or catheterization suite of constriction, we are confident in assuring the surgeon that there is no reason to take the patient to surgery. On the other hand, despite absence of constriction by other imaging modalities including the presence of a thin pericardium on computed tomography (CT), if there is no slippage in the major portion of the pericardium by CMR, we can assure the surgeon
that they will find an adherence pattern consistent with constrictive pericarditis upon opening the chest. In our series of more than 50 patients, we have never been incorrect.
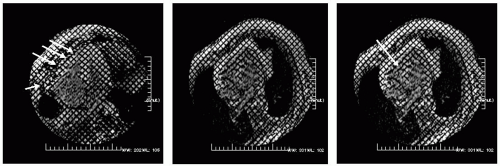
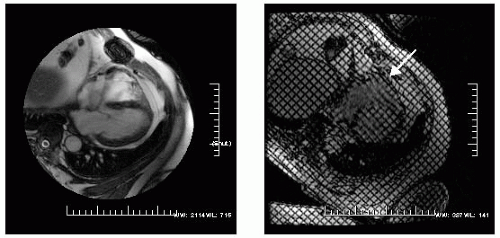 FIGURE 16-1 Steady state free precession (SSFP) in a four-chamber view (left) demonstrating a normal thickness pericardium and a sternal wire artifact. On the right is a radiofrequency tissue tagging image with the arrow pointing at the visceral-parietal interface. Note the distortion of the tagging pattern, indicating adherence between the two layers. By observing normals, an understanding of the typical interaction between the visceral and parietal layers can be appreciated while permitting a better understanding of the range of abnormal possible. The radiofrequency tissue tagging sequence is a standard option on all vendor protocols. Images relate to case study 1. |
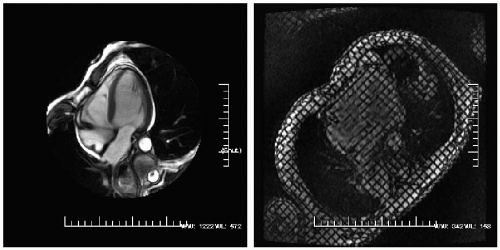 FIGURE 16-3 A steady state free precession (SSFP) four-chamber view demonstrated a small pericardial effusion (left) with a radiofrequency tissue tagging image (right) demonstrating normal pericardial slippage as expected between the two clearly separate pericardial layers. Images relate to case study 3. |
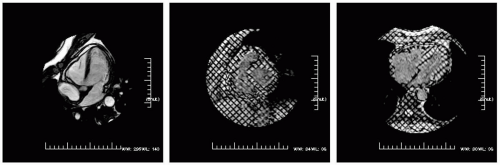 FIGURE 16-4 A steady state free precession (SSFP) (left) modified four-chamber view demonstrating a trace pericardial effusion, around the distal right ventricular free wall and left ventricular (LV) apex. Radiofrequency tissue tagging images in the short axis (middle) and four-chamber view (right) depict limited slippage between the visceral and parietal pericardium. Images relate to case study 4. |
CASE STUDIES
Case Study 1.
A 64-year-old man status post CABG presents with mild chest pain (CP) for which echocardiography revealed an abnormal right ventricular (RV) free wall motion. CMR demonstrates a normal pericardial signal throughout the entire left ventricular (LV) with the exception of the most anterobasal RV free wall, whereby the signal is no longer homogeneous (see Fig. 16-1, left). Tissue tagging (right panel) demonstrates focal adherence, denoted by deformation of the basal RV free wall, indicative of focal lack of slippage between the visceral and parietal pericardial layers (arrow) with normal slippage otherwise noted. Because this is the only abnormal region, this is regarded as a normal finding, and not as evidence of constrictive anatomy. The perfusion sequence (not shown) reveals a dense inferior septal hypoperfusion defect that becomes nearly circumferential toward the apex. Ten years following emergency bypass, all three saphenous vein grafts (SVGs) showed evidence of moderate occlusion, for which he underwent rebypass, following confirmation of the lack of constrictive pericarditis.
Case Study 2.
Adherence between the visceral and parietal RV pericardium in a 76-year-old status post CABG 2 years ago (see Fig. 16-2). Note, the normal thickness of the pericardium (best seen in middle panel) and deformation of the radiofrequency tissue tagging stripes. These signs are indicative of an adherence between the two pericardial layers (visceral and parietal). Upon surgical pericardiotomy, the noninvasive diagnosis was confirmed. We use this technique preferentially to volume loading in the catheterization suite or to ventricular interdependence in the echocardiography laboratory. To date, we have not been proved incorrect: if there is slippage seen between the two pericardial layers, the diagnosis of constrictive pericarditis (constriction) is excluded. The left panel demonstrates normal slippage for comparison (well seen along the RV).
Case Study 3.
A 50-year-old white woman with CP was sent for CMR evaluation (see Fig. 16-3). A very small pericardial effusion (bright) is present, which by use of radiofrequency tissue tagging, reveals no evidence of constriction (there is no adherence between the visceral and the parietal pericardium and there is a clean slippage plane). There is no evidence of tamponade physiology due to lack of right ventricular (RV) compression, lack of septal bounce, and no RA inversion.
Case Study 4.
A 65-year-old white woman with recurrent pericardial effusions (see Fig. 16-4). CMR demonstrates no effusion, but limited excursion between the visceral and parietal pericardium is seen focally in the lower left along the right ventricular outflow tract (RVOT) and anterior RV free wall (note the clean slippage plane through systole) best seen in right panel.
Case Study 5.
Standard double inversion recovery sequences obtained in a 54-year-old, demonstrating in axial and parasagittal images, a widely patent left main, proximal, and middle left anterior descending and proximal left circumflex vessels (see Fig. 16-5).
Case Study 6.
Case Study 7.
Two-dimensional (see Fig. 16-7A) right coronary artery (RCA) and 3D surface rendered imaging of the same vessel in a 54-year-old man with ST depression in the inferior leads suggestive of RCA ischemia.
Case Study 8.
A 67-year-old man, 1-month status post CABG presents with CP. Typically, a cardiac catheterization would be considered. However, the referring cardiologist felt the CMR breath-hold angiogram might be sufficient (see Fig. 16-8). Shown are of the 3D reconstructed images of a 22-second acquisition, demonstrating three widely patent saphenous vein grafts.
Case Study 9.
The double inversion recovery images in a 38-year-old man who presented with atypical CP (see Fig. 16-9). Cardiac catheterization revealed an anomalous origin of the RCA. Although unusual for the RCA to travel in the intra-arterial course when originating from the left coronary cusp, imaging was performed demonstrating the posterior course of the RCA, which finally emerged and travel in the usual trajectory along the atrioventricular (AV) groove.
Case Study 10.
A cardiac catheterization was performed on a 41-year-old man who presented with shortness of breath and an enlarged right heart by echocardiogram. The catheterization revealed an unusual directed anteriorly vessel, arising directly, and running superiorly from, the left anterior descending artery (LAD). The mid and distal course of the vessel was unknown, prompting CMR for further valuation (see Fig. 16-10). Seen in these SSFP sequences is a vessel traveling superiorly to, and anteriorly around the RVOT to empty into the supravalvular pulmonary artery. The course of this vessel is circuitous, taking a very serpentine pathway as it finally drains into the pulmonary artery. This prominent pulmonary artery fistula was quantified by Phase velocity mapping (PVM) to have a Qp:Qs of 1.3:1, which is a very small left-to-right shunt. Incorporating short-axis imaging of the right and left ventricles, the shunt was confirmed to be small. However, the RV was at the top of normal size. He was offered prophylactic coiling of the coronary fistula but the patient decided against any intervention.
Case Study 11.
A 32-year-old man presents with typical CP prompting a cardiac catheterization. The angiogram reveals a common ostium for all three of the coronary vessels. Clarification of the etiology, as well as determination of the trajectory of the anomalous vessels is performed by CMR (see Fig. 16-11). No single view reveals the common ostia of all three vessels, but through triangulation, it is clear that there is either a large ostia, or an adjacent, but near contiguous large ostia for all three vessels. In the left panel, the RCA is visualized whereas the mid and right panel depicts the left circumflex artery. The mid panel reveals the large common ostia for both the LAD and circumflex arteries traveling in separate directions. The LAD is seen in the right panel, derived from the common ostia (right arrow) whereas the left arrow signifies the RCA. This is an extremely rare congenital anomaly, whereby essentially all three vessels are derived from one common ostia, which is the right coronary cusp.
Case Study 12.
A 62-year-old white man with transient ischemic attacks (TIA) presented for transesophageal echocardiography (TEE) unable to be intubated. Subsequently, he was found to have esophageal strictures that were not amenable to Hagar dilation. Because he was not in normal sinus rhythm (NSR), exonerating his left atrial appendage (LAA) as the cardiac source of embolus was important; therefore a CMR was ordered (see Fig. 16-12). The LAA is seen in the SSFP with the pectinate muscles shown, despite a respiratory artifact from his inability to breath-hold. A 2 NEX scan with a 196 × 196 matrix was employed. PVM demonstrated a transappendageal emptying velocity of 50 cm per second supporting the lack of visible thrombus or a favorable milieu for its subsequent formation. The patient was not placed on Coumadin.
Case Study 13.
A73-year-old woman presents with a CVA as an esophageal stricture not amenable to intervention. A para-axial (see Fig. 16-13, left panel) as well as a two-chamber view (right) demonstrate a normal size for the LAA with normal contraction. The left panel reveals small pectinate muscles without any evidence of thrombus.