Species
Mouse
Man
Weight
20 g
70 kg
Blood volume
2 ml
5 l
GIT transit
20 h
40 h
Heart beat
600/min
65/min
Respiration
160/min
12/min
Renal blood flow
75 %/min
15 %/min
2 Receptor Expression
Most therapeutic radiopharmaceuticals localise in tissues as a result of an interaction with a molecular target which is expressed at higher levels on tumour cells than in normal tissues. The suitability of an animal model for predicting effects in humans, therefore, depends on the degree of conservation between these targets in the two species. Many receptors are highly conserved and a radioligand would be expected to bind equally well to the targets in both species. However, some are not identical and a radiopharmaceutical which has been optimised to bind in the animal model may work less well in man. For example, the bombesin analogues Demobesin 1 and Z-070 showed similar binding affinities to the GRP receptor in AR42J rat pancreatic tumour cells but Demobesin 1 showed 11–15 times higher binding affinity to Z-070 on the human prostate cancer–derived cell line PC-3 (Maina et al. 2005). Even those receptors with similar structures to man may well show a different tissue distribution. For example, the mouse pancreas expresses high levels of several neuropeptide receptors but these are often absent in man. Some targets used for molecular radiotherapy are generally lacking in the mouse model unless deliberately introduced by transgenic approaches, for example the epitopes recognised by monoclonal antibodies used for radioimmunotherapy.
3 Tumour Biology
Since most applications of molecular radiotherapy are directed towards treatment of cancer there is often a need to use tumour-bearing animals. Several types of tumour-bearing animals are available, including syngeneic, xenogeneic and genetically modified models and each of these have their own inherent advantages and disadvantages especially with regard to their ability to predict likely outcome in patients (Table 2).
Table 2
Mouse models in cancer research: characteristics, strengths and shortcomings in implementation and clinical efficacy prediction
Type | Material | Site | Advantages | Drawbacks |
|---|---|---|---|---|
Syngeneic | Chemical induction-injection of cells or explants from the same species | Predominantly subcutaneous; orthotopic | Immunocompetent hosts; availability of well-characterised cell lines; low cost; ease of implementation; reproducibility of tumour properties; simple statistics | Often poor representation of human disease; sometimes lack of target-molecule homology between species |
Xenogeneic | Injection of human cell lines or explants | Subcutaneous (unnatural site) | Availability of well-characterised cell lines; simple to implement; expression of human homologue of the target; efficacy data bases available; reproducibility; homogeneity in tumour characteristics | Immunosuppressed and non-human hosts; more costly and microbe-free animal housing; murine peritumoural milieu; different to human tumour histology |
Orthotopic (primary tumour source site) | Best mimicking human carcinogenesis and metastatic patterns | Complex logistics, surgical skills; limited number of hosts; non-homogeneity/non-reproducibility in tumour growth rates; problems in statistics | ||
GEM | Expression of target or “label”–spontaneous carcinogenesis | – | Controlled cancer progression in selected organs; resemblance of human carcinogenesis; immunocompetent syngeneic host | Limited availability of hosts; restricted experience; cost; variations in tumour growth rates; demanding statistics; murine host stroma |
In syngeneic models, mice bear tumours originating from their own species. Carcinogenesis is induced by means of chemical or surgical intervention; subsequently, material from this first tumour (either explants or cells) is introduced to naïve members of the same mouse strain. Advantages of syngeneic models are ease of implementation, availability of hosts at a low cost, reproducibility in experimental tumour histology and growth rate and simple statistical analysis required for data validation. In addition, the host retains full immunocompetence and tumour induction is not immunogenic. However, syngeneic models often fail to adequately represent the human situation. As modern medicines are directed to specific cancer-residing targets, the homology between the mouse and human versions of target biomolecules may turn out to be a serious limitation for syngeneic models.
Alternatively, xenografts of human origin can grow in immunosuppressed hosts, such as in genetically manipulated athymic mice (nu/nu), or in severe combined immunodeficiency (SCID) mice which lack both humoral and cellular immune components (Sausville and Burger 2006). Tumour induction is usually triggered by injecting human tumour material in the host either subcutaneously (sc) or orthotopically (vide infra). Subcutaneous xenogeneic models have been the mainstay of anticancer drug development in the last 25 years, mostly because they are better predictors of drug efficacy to human tumours. Malignant cells are human and consequently express the human homologue of the target biomolecule. However, the microenvironment around the tumour is provided by the non-human host. Discrepancies may arise in tumour histology and intra- and peritumoural vasculature as a result of altered interaction patterns between the human tumour and the extracellular matrix of the murine host. These differences can be minimised when human tumours are directly transplanted from patients into mice, whereby the xenograft histology closely resembles the histology of the patient tumour. In this case, the tumour cells instead of the host stroma seem to dictate lesion architecture, molecular features are preserved and existing human proangiogenic factors will “cooperate” with elements of the host peritumoural micromilieu in the process of neovasculature formation.
Subcutaneous xenogeneic models have dominated anticancer drug research also as a result of their simplicity, reproducibility and homogeneity in tumour histology and growth rates. In addition, a wide range of well-established human cell lines have long been available to researchers and drug efficacy databases have been created over the years. Hosts are also widely available, albeit more costly than normal mice. In view of their immunodeficiency, mouse hosts are susceptible to infections and need to be housed in a microbe-free environment. Still, the stromal component of model tumours remains murine and, most importantly, tumours grow at an unnatural site.
Unlike the subcutaneous model, in the orthotopic model human tumour material is introduced at the site of the primary tumour source (Bibby 2004). Accordingly, the xenograft grows in the tissue of origin of the primary tumour and might more faithfully mimic human carcinogenesis and later metastatic events. Given that skillful surgical intervention is often needed for tumour implementation, the number of available surgically manipulated mice will be limited. Disease onset and dissemination patterns may also vary not only between mice and men but also among mice, thereby further complicating execution of individual tests and statistical data validation. In contrast to patients, where primaries are as a rule surgically removed and morbidity is caused by metastatic disease, mice succumb to primary lesions well before disease spread has occurred. Thus, additional surgical intervention is frequently required to excise the primary lesion, further complicating the implementation of orthotopic models.
Despite the wide availability of human cell lines expressing a certain target molecule, naive cell lines are also genetically being manipulated to express molecular targets of interest. In a similar approach, they can be programmed to express a foreign “label” protein with the aim to monitor tumour induction and propagation in the host. Manipulations of the host genome may also result in spontaneous carcinogenesis in the immunocompetent syngeneic host. Transgenic mice may be engineered by exchange of the endogenous sequence of a gene to closer mimic human carcinogenesis (“knock-in” mice); alternatively, they may be genetically manipulated by disruption of a gene to suppress its function (“knock-out” mice). Today, transgenic mice may develop cancers in a diversity of organs with an inherently controlled progression closely resembling human carcinogenesis. Tumours develop spontaneously and have histological similarities to human tumours with which they share many molecular and genetic traits. However, the use of genetically engineered mice (GEM) has been limited in translational research of new therapeutics not only as a result of their cost and limited availability, but also because of logistic and practical issues (Hansen and Khanna 2004). For most healthy animals, including humans, cancer is a disease of old age, occurring after a lifetime of DNA damage, which results in the loss of replication control in some cells. Testing chemicals for cancer induction potency is challenged by the tendency for many test species to develop spontaneous tumours late in life, as well as by the number of animals surviving long enough for tumour induction to be observed. As the period of cancer onset and subsequent metastatic spread (known as the “risk period”) can greatly vary between members of the same group of GEM, it becomes practically challenging to conduct a statistically tight evaluation study.
4 From Mouse to Man: Radiation Dose Distribution
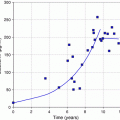
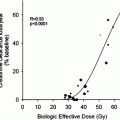
One major difference between mouse and man is size and this has a profound effect upon the distribution of energy deposited by radionuclides localised at a particular site. It follows that, especially for higher energy beta particles such as those emitted by yttrium-90, energy will be delivered to many normal tissues surrounding the tumour or other site of uptake of the radiopharmaceutical. However in some situations, even the dose distribution patterns of radionuclides with low pathlengths will be very different in mice to those seen in man. For example, the diameter of the mouse spine is only around 2 mm while that of man is around 5 cm. Even some internal conversion electrons emitted by radionuclides localised to the bone mineral will, therefore, deliver a significant radiation dose to the bone marrow while this will be negligible in man.
The combination of differences in biodistribution of radiopharmaceuticals and organ geometry between mouse and man means that major differences would be expected between the two species when radiation doses to various tissues are calculated. In fact, few direct head-to-head comparisons of radiopharmaceuticals have been performed but a few examples can be gleaned from the literature as shown in Tables 3, 4, and 5.
Table 3
Radiation doses from 90Y-J591 (mGy/MBq)
Mouse (Vallabhajosula et al. 2004) | Human (Pandit-Taskar et al. 2008) | ||
|---|---|---|---|
Tissue | Dose | Tissue | Dose |
Blood | 33 | Whole body | 0.5–0.6 |
Bone marrow | 12 | Red marrow | 0.6–0.9 |
Liver | 2.5−5.7 | ||
Tumour | 49 | Lesion | 58–97 |
Table 4
Radiation doses from 90Y-hLL2
Mice (Griffiths et al. 2003) | Humans (Juweid et al. 1999) | ||
|---|---|---|---|
Liver | 19 | Liver | 0.04 |
Spleen | 14 | Spleen | 0.10 |
Kidney | 20 | Kidney | 0.03 |
Lung | 19 | Lung | 0.03 |
Stomach | 9 | ||
Sm Int | 10 | ||
Lg Int | 5 | ||
Bone | 10 | ||
Red marrow | 0.02 | ||
Table 5
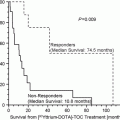
Radiation doses from 177Lu-DOTA-Tyr3-octreotate (mGy/MBq)
Dose in rats calculated from
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||
|---|---|---|