Fig. 1.
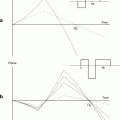
In vivo 3D acquisition of a mouse brain: sagittal (top), coronal (left), and axial (right) views. Cerebral ventricles filled with CSF appear hyperintense on these images (arrows). The measure of their volumes can be used as an index of atrophy.
MR methods using MRI are under development to either quantify some parameters that reflect amyloid load (11, 12) or to directly detect amyloid plaques (13, 14).
This chapter describes methods to detect amyloid plaques in vivo and ex vivo in the brains of transgenic mouse models of AD.
2 Material
2.1 Mouse Models
APP/PS1dE9 mouse models of AD can be purchased from the Jackson Laboratory (http://www.jax.org/; 600 Main Street Bar Harbor, Maine 04609 USA) (see Note 1).
2.2 MR Imaging Systems for In Vivo Imaging
1.
MRI spectrometer and MR probes: 7-T spectrometer (Pharmascan, Bruker Biospin GmbH) equipped with a 9-cm inner diameter gradient system (760 mT/m strength and 6836 T/m/s slew rate) and interfaced to a console running Paravision 5.0. A birdcage coil (Bruker) of 38 mm diameter can be used for power transmission–reception. Other high-field MRI systems can also be used for in vivo imaging of mouse models of AD.
2.
Mouse holder and monitoring (Fig. 2): the head of the animal is stabilized in a head holder using ear bars and a bite bar built in a dedicated cradle (available from RAPID Biomedical GmbH, Germany). The head holder is inserted into the radio frequency (rf) coils during the imaging session. This setup prevents movements of the animals during the long imaging acquisitions. Monitoring devices, such as the MR-compatible small animal monitoring and gating system (respiration/IBP module) from SA Instruments Inc (Stony Brook, NY 11790, USA), are used to follow the animal’s physiological parameters. The animals are warmed with a water-filled heating blanket connected to a thermoregulated water bath (circulating thermostat system from Bruker).
3.
Anesthetic devices: isoflurane vaporizer connected to several flowmeters for O2/N2O/Air and one cage dedicated to anesthesia induction (Minerve, Esternay, France).
4.
Isoflurane gas (Belamont, Paris, France) is used for anesthesia.
5.
Air, O2, N2O tanks.
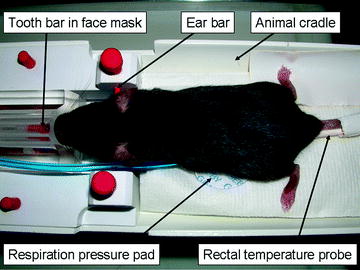
Fig. 2.
Mouse setup for in vivo MR imaging. The animal is held still with tooth and ear bars. The isoflurane anesthesia is delivered via a face mask. Respiration is recorded through a pressure pad and body temperature is recorded through a rectal probe.
2.3 Animal Sacrifice
1.
Peristaltic pump for intracardiac perfusion: Mini-peristaltic pump II by Harvard Apparatus (Fig. 3).
2.
Surgical supplies such as scissors (available from WPI Europe (http://www.wpi-europe.com), dressing forceps (10 cm long), micro bulldog clamp (3 cm long), butterfly needle (25 gauge).
3.
Perfusion fluids: phosphate-buffered saline, and 10% buffered formalin at room temperature.
4.
Surgery board and draining bucket.
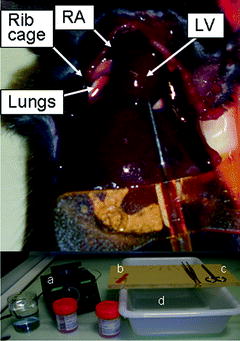
Fig. 3.
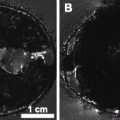
Mouse intracardiac perfusion. The top picture shows the rib cage cut off and the butterfly needle inserted into the left ventricle (LV). The bottom picture shows the material used: formalin beakers and PBS beaker, peristaltic pump (a), butterfly needle (b), forceps, scissors (c), surgical board and draining bucket (d). RA, right atrium.
2.4 MR Imaging Systems for Ex Vivo Imaging
1.
Clinical 7-T spectrometer (Syngo MR, VB15, Siemens), equipped with an AC84 head gradient set with 36-cm available bore (80 mT/m strength and slew rate of 333 mT/m/s). Birdcage coils can be used (inner diameter = 24 mm) for signal transmit–receive. Other MR systems can also be used for ex vivo MRI. For example, we performed previous studies on a 4.7-T spectrometer (Bruker) (13) or on the 7-T spectrometer used for in vivo imaging.
2.
10-mL syringes can be used to make containers that allow keeping the brain still in place and soaked.
3.
Fluorinert™ Electronic Liquid FC-40(3 M™, Cergy-Pontoise, France), a fully fluorinated liquid is used to embed the sample before MRI and to remove any background signal.
2.5 Histological Analysis
2.5.1 General Histology
1.
Sliding freezing microtome (e.g., LEICA SM2400).
2.
Homemade baskets to rinse tissue. Commercial systems (15-mm Netwell insert with 74-μm mesh size polyester membrane from Corning Life Sciences) are available as an alternative.
3.
Slow orbital agitator and routine small equipment for histology lab.
4.
Phosphate buffer (PB).
5.
Dimethyl sulfoxide.
6.
Glycerol.
7.
Dry ice.
8.
Superfrost+ glass slides.
2.5.2 β-Amyloid Staining with BAM10 Antibody
1.
Usual glassware and small equipment for histology lab.
2.
Phosphate-buffered saline (PBS).
3.
Hydrogen peroxide 30% (Sigma H 0904).
4.
Octylphenol ethylene oxide condensate 0.2% (Triton X-100™, Sigma).
5.
Normal rabbit serum, can be aliquoted at −20°C (Vector® Labs S 5000).
6.
Monoclonal BAM10 clone A3981 (Sigma).
7.
Biotinylated IgG anti-mouse, BA-9200 (Vector®).
8.
Sodium azide 8%, stored at room temperature (Sigma S 8032).
9.
Avidin–biotin complex (ABC VECTASTAIN kit, Elite PK 6100).
10.
Tyramin biotin reagent, stored at 4°C (Blast PC 2815-0897)
11.
Peroxidase substrate kit (VIP SK 4600 substrate kit for peroxidase, Vector®).
2.5.3 β-Amyloid Staining with Congo Red
Labeling of amyloid deposits is done by standard Congo red staining (adapted from Ref. (15)).
1.
Usual glassware and small equipment for histology lab.
2.
Solution S1: 80° ethanol saturated with NaCl.
3.
Solution S2: Saturated solution of Congo red (Fluka, Ref. 60910) made in saline ethanol.
S1 and S2 solutions are stable for weeks/months but it is recommended to prepare them immediately before staining.
4.
Sodium hydroxide.
2.5.4 Iron Staining with Perls-DAB Method
Staining of iron deposits is performed by means of the Perls’ method with diaminobenzidine intensification (16).
1.
Usual glassware and small equipment for histology lab.
2.
Potassium ferrocyanide (Sigma ref. P 9387).
3.
Tris buffer.
4.
Diaminobenzidine (DAB) dissolved in distilled water (1 g/1000 ml). DAB solution can be prepared and aliquoted at −20°C before use.
5.
Methanol.
6.
Hydrogen peroxide 35%.
7.
Hydrochloric acid 35%.
2.5.5 Analysis and Quantification of Histological Stainings
1.
Slide scanner with high optical resolution (e.g., Super CoolScan 8000 ED scanner, Nikon, Champigny sur Marne, France) (see Note 2). Use a scanner that has a 4000 dpi in-plane digitization resolution (pixel size 6.35 μm2) to allow quantification of large objects (e.g., plaques and big focal iron deposits). Work under calibrated and constant illumination conditions.
2.
Optical microscope equipped with a digital camera (optional).
3.
ImageJ freeware (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2005).
4.
Adobe Photoshop® software or any image manipulation program (e.g., The Gimp, http://www.gimp.org/, can be a good freeware alternative).
3 Methods
3.1 Mouse Preparation for In Vivo MRI
1.
Place the mouse in the induction cage.
2.
Turn the oxygen or air tank on.
3.
Turn the flowmeter up to 1–1.5 L/min.
4.
Induce anesthesia by turning the isoflurane level to 5% until the animal is in lateral decubitus for 2 min.
5.
Maintain anesthesia at a concentration of 1.0–1.5% (see Note 3).
6.
Place the mouse prone in the animal cradle of the MR scanner; insert the teeth into the tooth bar and the ear bars into the ear canal; put the respiration and temperature probes in place; cover the animal with the warming blanket.
7.
Insert the head of the animal in the rf coil and slide the animal into the magnet for imaging.
3.2 In Vivo MR Brain Imaging
The parameters for a typical T 2-weighted spin echo sequence are presented in Table 1. The resolution with these parameters is about 117 μm isotropic and the acquisition time is about 42 min.
Table 1
Example of acquisition parameters for the fast spin echo sequence used to record T 2-weighted MR images in vivo
Parameter | Value | Unit | |
|---|---|---|---|
Repetition time | TR | 2500 | ms |
Echo time | TE | 92.3 | ms |
Field of view | FOVx FOVy FOVz | 15 30 15 | mm |
Matrix | MATx MATy MATz | 128 256 128 | |
Rare factor | 16 | ||
3.3 Mouse Sacrifice and Preparation for Ex Vivo MRI
3.3.1 Mouse Sacrifice and Fixation
The entire perfusion fixation procedure should be performed under a hood, possibly equipped with a sink connected to ad hoc disposal to evacuate perfused fluids.
1.
Prepare the pump: pour about 500 mL of PBS in a beaker and 500 mL of formalin in another beaker. Connect a butterfly needle to the outflow end of the peristaltic pump and drop the inflow end of the pump into the PBS beaker. Run the pump until no more air bubbles are visible in the perfusion line.
2.
Anesthetize the mouse with an intraperitoneal injection of pentobarbital sodium (at a dose of 100 mg/kg).
3.




When the animal is deeply anesthetized and all reflexes are lost (toe- and tail-pinch checks), place the animal in supine position on a dissecting board placed over a draining bucket. Tape or pushpin the limbs away from the body to hold the animal still. Proceed fairly quickly to begin the perfusion before the heart stops beating.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree