Current Practices in Active Surveillance
Active surveillance is a management strategy designed to identify prostate cancer patients who are low risk of cancer progression and to intervene with active treatment only when disease progression is identified at follow-up.
Eligibility varies widely across different AS programs, incorporating criteria based on PSA level, PSA density, clinical T stage, Gleason score (GS), number of positive cores, and percentage of cancer involvement in each core. 21 For example, in the Johns Hopkins cohort, eligibility is defined by the Epstein criteria: clinical stage T1c, PSA density ≤ 0.15 ng/mL/mL, GS ≤ 6, ≤ 2 biopsy The space has been drained out of this line making this very important list of criteria very hard to read and absorb. Please reinstate spacing around units of measure and make this readable again. cores with cancer, and maximum of 50% involvement of any core with cancer. This definition is purposefully narrow in order to include only those patients who are least likely to undergo subsequent pathologic reclassification or, worse, tumor spread. However, many protocols, including those from the European Prostate Cancer Research International Active Surveillance (PRIAS) program, University of California San Francisco, and University of Toronto, include clinical stage T2 patients (i.e., patients with a positive digital rectal examination [DRE]). 22, 23, 24 Moreover, other protocols include patients with GS = 7 and meeting National Comprehensive Cancer Network (NCCN) criteria for intermediate-risk disease. 25, 26
Just as eligibility varies widely between AS protocols, so do monitoring strategies. Progression among men on AS is defined by reclassification into a higher-risk group. This reclassification is traditionally determined by progression based on any of a number of factors, including PSA kinetics (i.e., PSA velocity or doubling time beyond a certain threshold), Gleason grade reclassification, tumor volume reclassification (i.e., increased percentage of tumor involvement within positive cores or increased number of cores that are positive), and T stage progression (i.e., palpable abnormality on DRE). More recently, either an increasing tumor size or a more worrisome appearance of the tumor on MRI as well as worsening genetic features have been proposed as triggers for intervention. 27 Each monitoring approach has its own risks and benefits. For example, while utilizing solely PSA kinetics avoids the costs and morbidity that are associated with annual prostate biopsy, such an approach would lead to misclassification in 12% of patients in the Johns Hopkins cohort. 28 Thus, PSA kinetics are currently being replaced with MRI as a way to monitor patients on AS and avoid annual biopsy.
Despite differences among protocols, long-term follow-up of AS cohorts has consistently demonstrated overall favorable outcomes. Nonetheless, different eligibility and monitoring strategies confer different risks of disease reclassification, progression to metastasis, and prostate cancer–related death. In a study from the University of Toronto by Klotz et al, patients with low-risk and favorable intermediate-risk (Gleason score 3+4 and/or PSA level of 10–20 ng/mL) disease were enrolled and monitored with a combination of PSA testing every 3 to 6 months and biopsies every 3 to 4 years. This enrollment and monitoring protocol resulted in 28 of 993 patients (2.8%) progressing to metastasis at a median time of 7.3 years after the initial biopsy. 29 While the majority of patients died of other causes (particularly cardiovascular disease), 15 patients (1.5%) died from prostate cancer. A disproportionate number of patients who were reclassified and progressed had Gleason score 3+4 disease at the time of enrollment. In comparison, in a Johns Hopkins cohort of 1298 men, enrollment was limited to patients with low-risk and very low-risk (i.e., all having Gleason score 6) disease, and men were monitored with annual biopsy. 8 In that conservative protocol, metastasis-free survival was 99.4%, with just two deaths from prostate cancer (a 99.9% cancer-specific survival rate).
10.3 Multiparametric MRI of the Prostate: Technical Considerations
Multiparametric MRI (mpMRI) of the prostate combines morphological and functional assessments of intracellular and intercellular environments and tissue perfusion. In patients with small volume low-grade prostate cancer, cellular alterations and associated diffusion and perfusion abnormalities may be subtle and thus difficult to detect using mpMRI. On the other hand, in patients whose presumed low-risk disease was undersampled and in fact harbor intermediate- or high-risk cancer, mpMRI can reliably detect lesions that were not adequately sampled during systematic TRUS-guided biopsy, often in locations such as the anterior transition zone and the apex. These larger-volume, higher-grade cancers are typically visible on mpMRI and display characteristic features: (1) low signal intensity replacing the hyperintense background of normal prostate on T2-weighted imaging (T2WI); (2) restricted diffusion on diffusion-weighted imaging (DWI) due to the high cellular density and extracellular disorganization; (3) alterations in the tumor microvasculature leading to perfusion abnormalities on dynamic contrast-enhanced MRI (DCE-MRI) and (4) elevated choline levels on MR spectroscopic imaging (MRSI). Thus, mpMRI may improve the initial risk stratification of men with newly diagnosed prostate cancer by minimizing the overdiagnosis of insignificant disease and reliably detecting high-risk disease. In a similar fashion, mpMRI may facilitate more reliable monitoring of men enrolled in AS.
In one study, a combination of parameters derived from T2WI, DWI, and DCE-MRI was reported as comprising the optimal strategy for imaging low-risk prostate cancer in the peripheral zone, having a sensitivity and specificity of 85% and 83%, respectively. 30 For the detection of low-risk disease in the transition zone, the combination of T2WI and DWI, but without DCE-MRI (which exhibits highly overlapping features with benign prostatic hyperplasia in this zone), offered the highest sensitivity and specificity at 88% and 86%, respectively. 30 This combination of MR parameters was more beneficial for the detection of intermediate- and high-risk disease than for the detection of low-risk tumors in the transition zone. 31 In addition, several studies assessed correlations between MR parameters and tumor grade. For example, Tamada et al evaluated the apparent diffusion coefficient (ADC) derived from DWI as a predictor of prostate cancer grade on histopathology. 32 ADC values in peripheral zone tumors exhibited significant negative correlation with tumor Gleason score (r = 0.497). 32 Similarly, Doo et al reported that the mean ADC value of tumors with a Gleason score of 7 or higher (< 800 × 106 mm2/s) was significantly lower than that of tumors having a Gleason score of 6 (> 800 × 106 mm2/s). 31 In comparison, quantitative parameters derived from DCE-MRI have not been shown to correlate with the grade or with vascular endothelial growth factor (VEGF) expression as a molecular marker of angiogenesis. However, one DCE-MRI parameter, the contrast agent backflow rate constant (kep) (washout), was positively correlated with the mean blood vessel count and the mean vessel area fraction estimated from prostate cancer (r = 0.440 and 0.453, respectively) in a study by Oto and colleagues. 33 Finally, the diagnostic performance of MRSI for tumor detection and grade prediction has been variable due to the complexity of scanning protocols and postprocessing of spectral data. MRSI tends to show improved performance for higher Gleason score tumors. In a study by Zakian et al, MRSI had higher sensitivity of 89.5% for detection of tumors with a Gleason score of 8 or above compared to a sensitivity of 44.4% for detection of low-grade tumors (Gleason score of 6). 34 A modest correlation between metabolite ratio and tumor grade was documented, with the mean choline and creatine–to–citrate ratio (CC/C ratio) discriminating low-grade tumors from higher-grade tumors that would not be eligible for AS. 34There are several technical considerations when imaging men with small-volume prostate cancer. The 3-T whole-body MR scanners are increasing in availability and offer increased signal-to-noise ratio (SNR) and the potential for substantial improvements in spatial, spectral, and temporal resolution. The application of an endorectal coil (ERC) allows further increases in spatial resolution for morphological assessment, which may be especially useful for staging, in temporal resolution for DCE-MRI, as well as in spectral resolution for MRSI. 35 Although ERC is recommended at a standard clinical field strength of 1.5 T to obtain a sufficiently high SNR and adequate spatial resolution, the need for an ERC for the detection or localization of prostate cancer has not yet been resolved at the higher–field strength of 3 T. Advocates for imaging with an ERC argue that integrated dual-coil prostate MRI (using both ERC and pelvic coil) detects more cancer foci than nonendorectal-coil MRI, with reported sensitivities of 0.76 and 0.45 and positive predictive values of 0.80 and 0.64 for the dual-coil and nonendorectal-coil approaches, respectively. 36 The mean size of detected lesions with nonendorectal-coil MRI was larger than that of lesions detected by dual-coil MRI (22 mm vs. 17.4 mm, respectively), suggesting more reliable detection of small lesions when using an endorectal coil. 36 On this basis, it has been suggested that adding MRI using an ERC to the initial clinical evaluation may be useful for achieving the most accurate assessment of eligibility for an AS program. 37 The arguments against the use of ERC include the increased susceptibility artifact and signal intensity inhomogeneity resulting from the nonuniform ERC-sensitivity profile, necessity for proper ERC positioning in order to optimize the anatomical coverage, additional time needed for placement and position verification, patient discomfort and motion artifact, gland deformation, and additional cost. For instance, one study showed that at 3 T, ERC was not necessary to achieve high accuracy for the detection of significant prostate cancer. 38Given ongoing technological advancements along with standardization in prostate imaging reporting through the Prostate Imaging–Reporting and Data System (PI-RADS), 39 mpMRI, with its excellent soft-tissue contrast and ability to assess tissue diffusion and perfusion, offers a diagnostic tool to detect and characterize clinically significant prostate cancer in men managed by AS, including in the anterior gland and apex that are traditionally undersampled on TRUS-guided biopsies. MRSI findings allow individual-based risk stratification that can be employed for better evaluation of candidates for AS, as well as for the triage of AS patients for curative treatment when intermediate- and high-risk tumors are detected.
10.4 MRI Detection of Clinically Significant Disease
Men on AS protocols are thought to harbor favorable-risk disease, such that the role of MRI in these patients is to expose a more worrisome lesion. As traditional TRUS-guided prostate biopsy inadequately samples the anterior portion of the prostate, a major concern in placing low-risk patients on AS is the presence of a missed high-grade anterior lesion. 40, 41
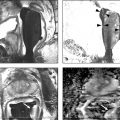
The location of prostate cancer within the gland affects its detectability on standard biopsy. In this regard, mpMRI has the advantage over TRUS-guided biopsy by allowing a detailed assessment of the entire gland ( ▶ Fig. 10.1; ▶ Fig. 10.2). As previously noted, intermediate-risk prostate cancer can be missed by TRUS-guided biopsy if located in the anterior transition zone ( ▶ Fig. 10.3) or at the apex ( ▶ Fig. 10.4). In a study by Komai and colleagues, 40% of patients (26 of 65) with a worrisome anterior lesion on MRI had negative prostate biopsies. 42 These anterior tumors are more likely to be large (> 1cm), and a subset of these tumors has high-risk features and an increased risk of extraprostatic extension. 43

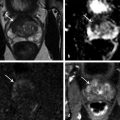
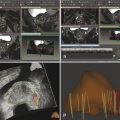
Fig. 10.1 A 71-year-old man with an elevated prostate-specific antigen (PSA) level of 4.83 ng/mL and multiple negative transrectal-ultrasound–guided (TRUS-guided) biopsies. Multiparametric MRI (mpMRI) at 3T without an endorectal coil demonstrates on axial T2-weighted image (a) a circumscribed, homogeneously moderately hypointense 10-mm lesion (arrow) in the left mid–peripheral zone. On the apparent diffusion coefficient map (b), the lesion is markedly hypointense (arrow). On dynamic contrast-enhanced MRI (c), there is focal early enhancement (arrow) corresponding to (a) and (b). Based on mpMRI, this lesion is scored as PI-RADS 4 (clinically significant cancer is likely to be present). A targeted biopsy with MRI-TRUS fusion (arrow) was performed (d) on a GE Logiq E9 system (GE Healthcare, Milwaukee, WI), demonstrating the MRI lesion to harbor tumor with Gleason score 4 + 4 = 8 involving 2 cores (30%, 40%). Note axial TRUS (left) and MR (right) images displayed side by side following anatomical coregistration with the biopsy needle (arrowhead) in the target lesion on the TRUS image.

Fig. 10.2 A 72-year-old man with a prostate–specific-antigen (PSA) level increase from 3.0 to 4.5 ng/mL and standard 12-core transrectal ultrasound–guided (TRUS-guided) biopsy showing tumor with Gleason score 3 + 3 = 6 in 5% of 1 core from the right base, as well as high-grade prostatic intraepithelial neoplasia in 3 other cores and atypical glands in 1 core from the left apex. Based on the TRUS-guided biopsy results, the patient has very low-risk prostate cancer and was deemed a candidate for active surveillance. However, the patient was anxious and multiparametric MRI (mpMRI) was performed. mpMRI at 3T with an endorectal coil shows on axial T2-weighted image (a) multiple hypointense foci (arrowheads) as well as a dominant 10-mm hypointense lesion (arrow) in the posterolateral left peripheral zone. The index lesion (arrow) abuts the capsule of the prostate with capsular irregularity and thus is at risk for extraprostatic tumor extension. On the apparent diffusion coefficient (ADC) map (b) all lesions are markedly hypointense (arrowheads), and the index lesion (arrow) exhibits an ADC value < 800 µm2/s while the neighboring peripheral zone exhibits ADC values above 1400 µm2/s. On dynamic contrast-enhanced MRI (DCE-MRI) (c), the index lesion shows early enhancement (arrow). The color-coded DCE-MRI (d) illustrates abnormal perfusion (red) in the dominant peripheral zone lesion (arrow) and within the additional lesions. Based on mpMRI, a multifocal prostate cancer was suspected with a score of PI-RADS 5 (clinically significant cancer is highly likely to be present). Patient underwent robot-assisted laparoscopic radical prostatectomy that showed a dominant Gleason score 4 + 3 =7 tumor in the left lateral and posterolateral peripheral zone. There was nonfocal extraprostatic extension and microscopic left seminal vesicle invasion. Surgical margins were negative. The case illustrates the role of mpMRI in reclassifying patients who may be erroneously categorized as having low-risk disease due to undersampling of high-risk tumor on standard biopsy.


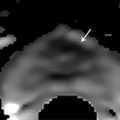
Fig. 10.3 A 65-year-old man with a slowly rising prostate-specific antigen level over 4 years from 2.98 to 6.95 ng/mL and with a negative transrectal ultrasound–guided (TRUS-guided) biopsy. Multiparametric MRI (mpMRI) at 3T without an endorectal coil demonstrates on axial T2-weighted image (a) a noncircumscribed, moderately hypointense 16-mm lesion (arrow) in the left anterior transition zone. On the apparent diffusion coefficient map (b), the lesion is markedly hypointense (arrow). On dynamic contrast-enhanced MRI (DCE-MRI) (c), there is focal early enhancement (arrow) corresponding to (a) and (b). The color-coded DCE-MRI (d) shows abnormal perfusion (red) in the left anterior transition zone lesion (arrow) extending to the anterior prostate margin and appearing asymmetric when compared to the enhancement within the right transition zone. Based on mpMRI, this lesion is scored as PI-RADS 5 (clinically significant cancer is highly likely to be present). A targeted biopsy with MRI-TRUS fusion was performed (e) using the UroNav system (Invivo Inc. (Phillips), Gainesville, FL), in which the target lesion (arrow) demonstrated Gleason score 3 + 3=6 tumor involving 3 cores (100%, 40%, 5%). Note axial TRUS (top left) and MRI (bottom left) images displayed following three-dimensional volumetric coregistration of TRUS and MRI datasets (red contours) with the biopsy needle trajectory marked (yellow) in the target lesion (green contour).

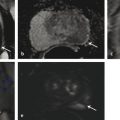
Fig. 10.4 A 71-year-old man with a prostate-specific antigen (PSA) level of 14.6 ng/mL (PSA density = 0.17) and with prior standard transrectal ultrasound–guided (TRUS-guided) biopsy showing Gleason score 3 + 3 = 6 prostate cancer in 20% of 1 core. Patient had urosepsis following the TRUS-guided biopsy. Multiparametric MRI (mpMRI) at 3T without an endorectal coil demonstrates on axial T2-weighted image (a) a large homogeneously moderately hypointense 28-mm lesion (arrow) in the apex, located anterior to the urethra. On the apparent diffusion coefficient (ADC) map (b), the lesion is markedly hypointense (arrow), exhibiting an ADC value < 800 µm2/s, which is suspicious for high-grade prostate cancer undersampled on the TRUS-guided biopsy. On dynamic contrast-enhanced MRI (c), there is focal early enhancement (arrow) corresponding to (a) and (b). Based on the mpMRI, this lesion is scored as PI-RADS 5 (clinically significant cancer is highly likely to be present). Given the results from mpMRI and PSA density, this patient was not a candidate for active surveillance and radiation therapy was recommended.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree