T2-weighted imaging
T1-weighted imaging
Parameter
Half-Fourier single-shot FSE
Half-Fourier single-shot FSE with fat suppression
Diffusion-weighted imaging
b800
2D dual-echo GRE
2D time of flight
Planes
Axial, coronal, sagittal
Axial
Axial
Axial
Axial
Coverage
Above gallbladder thru pelvis
Above gallbladder thru pelvis
Above gallbladder thru pelvis
Above gallbladder thru pelvis
Renal veins to pubic symphysis
FOV (mm)
360–440
300–360
360
300–360
300–360
TR (ms)
800–1100
800–1100
10,000
180–210
30
TE (ms)
60–80
50–70
55–80
2.2, 4.4
4.5–10
Slice thickness/intersection gap (mm)
4/1
4/1
5/0
5/1
3/1
NEX
1
1
1
1
1
Matrix
256 × 192
256 × 192
64 × 64
256 × 160
256 × 128
Breathing
Held
Held
Held
Held
Free
This protocol is both minimalist and thorough with each sequence serving a particular purpose. Multiplanar T2-weighted imaging (T2WI) with single-shot techniques is relatively motion insensitive and serves to best localize anatomic structures. Particularly in the pregnant population, these sequences are invaluable given fetal motion and potential limited breath-holding ability of the mother which can contribute to motion degradation of the study. T2WI with fat suppression and diffusion-weighted imaging (DWI) highlight bowel pathology with inflammation, edema, fluid, and focal lesions often appearing bright on these sequences. 2D dual-echo gradient echo T1-weighted imaging (T1WI) is used to characterize soft tissue lesions by assessing for the presence of hemorrhage, fat, and proteinaceous content which typically appears as hyperintense on this sequence, as well as for the presence of magnetic susceptibility from air, calcification, or hemosiderin depicted as blooming on the longer echo time sequence (in-phase sequence on 1.5 T magnet). Finally, 2D time of flight (TOF) is helpful in differentiating between the bowel and in particular the appendix from other vascular mimickers such as pelvic varices.
In optimal circumstances, image acquisition can be completed in approximately 15 min. Longer exam time may result with the use of respiratory-triggering or navigator techniques when the patient is unable to adequately hold their breath. Respiratory-triggered single-shot sequences have the added benefit of enabling sequential imaging of the bowel. Additional enterographic sequences can be considered when dynamic bowel evaluation is thought to be of benefit, as for a patient with known inflammatory bowel disease. These sequences include multiphase, multislice, cine imaging, acquired as heavily T2-weighted thick slabs, as well as coronal steady-state free precession (SSFP). The former allows for dynamic assessment of bowel peristalsis and distensibility, while the latter provides excellent anatomic detail due to motion insensitivity.
In order to expedite the imaging acquisition process, MRI obtained in pregnant patients with suspected appendicitis should be acquired without the use of oral contrast. Despite the controversial benefits of oral contrast agents, particularly an iron-containing negative contrast agent such as historically available ferumoxsil, recent literature has confirmed maintained high sensitivity and specificity of MRI in diagnosing appendicitis without its use [1, 7–9].
Intravenous gadolinium-based contrast agents are contraindicated in pregnancy and should only be used in dire situations in pregnancy, when the additional information it can provide outweighs the theoretical risks to the fetus [10]. Typically, bowel assessment can be sufficiently conclusive without the use of a gadolinium-based intravenous contrast, given the excellent diagnostic information which can be garnered from the remainder of the MRI examination [11].
23.3 Acute Appendicitis
Acute appendicitis remains the most common nongynecologic cause of abdominal pain during pregnancy requiring surgery [12]. The diagnosis of appendicitis in pregnancy can be complex due to the inaccuracy of conventional clinical parameters for this diagnosis [13]. Furthermore, a perforated appendix in pregnancy can result in extremely high consequences [14, 15]. Therefore, a reliable and accurate imaging method for the diagnosis of appendicitis is needed. Currently, in pregnant patients suspected of having appendicitis, the American College of Radiology recommends the use of MRI following an inconclusive ultrasound assessment of the appendix [16]. The appendix can be detected by MRI in 92 % of the cases [17], with excellent sensitivity and specificity for the diagnosis of appendicitis about 90 % and greater than 95 %, respectively [18–22], comparable to those of CT. Due to the extremely high negative predictive value of MRI for excluding appendicitis [1, 17, 23], and the ability of MRI to identify alternative causes of right lower quadrant pain in pregnancy [24], the addition of MRI to the workup of pregnant females with right lower quadrant pain has been shown to decrease the frequency of negative laparoscopies [1, 17]. The time-to-surgery delay from the addition of MRI has not been shown to increase the rate of appendiceal perforation, a critical complication [1, 17].
23.3.1 Normal Appendix
A normal appendix is a blind-ending tubular structure originating from the cecal tip. Accepted normal dimensions are a single wall diameter of 2 mm or less and a total cross-sectional diameter of 6 mm or less. A normal appendix is typically intermediate in signal intensity on T1- and T2-weighted images, similar to that of muscle (Fig. 23.1) [21]. While a normal appendix may be collapsed or partially filled with fluid and/or gas, the presence of gas throughout the appendiceal lumen indicates patency, thereby virtually excluding the diagnosis of appendicitis regardless of appendiceal diameter. T1-weighted in-phase and opposed-phase gradient echo imaging is useful in identifying luminal gas, denoted by susceptibility artifact on longer echo time in-phase images (on 1.5 T magnet) (Fig. 23.2) [7].
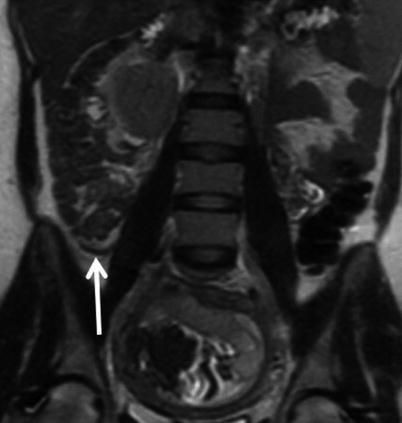
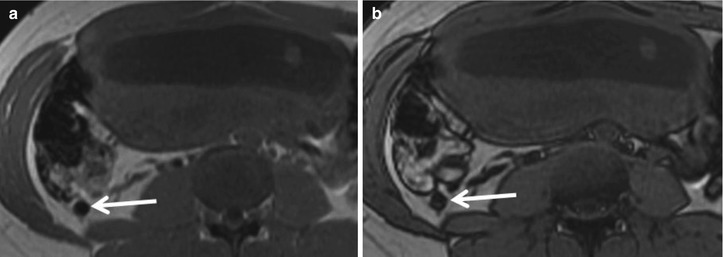

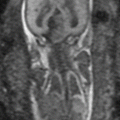
Fig. 23.1
Coronal T2-weighted single-shot FSE image of a 31-year-old female at 12-week gestation demonstrating a normal appendix (arrow)

Fig. 23.2
Axial T1-weighted in-phase (a) and opposed-phase (b) GRE images in a 28-year-old 20-week pregnant patient demonstrate susceptibility blooming artifact (arrow) associated with appendiceal intraluminal gas on the longer echo time in-phase image, confirming a normal appendix
Often the limiting factor in reaching conclusive MRI results is definitive identification of the appendix. Progressive displacement of the appendix by the gravid uterus and compression of adjacent structures can make this step quite difficult, depending on patient habitus and gestational age. With progressive gestational age, there is a general trend for upward migration of the appendix [25]. Regardless of gestational age, the cecal tilt angle can be used as a reliable way to localize the appendiceal base. Seen best on sagittal or coronal single-shot FSE images, identifying the cecum angled greater than 90° relative to the longitudinal axis of the patient places the appendiceal base within the right upper quadrant [25]. Tactics for identifying the cecum include retrograde tracing of the colon, as well as localization of the ileocecal junction, with the appendix typically originating along the same side of the bowel wall, approximately 2.5 cm distally.
23.3.2 Acute Appendicitis Imaging Features
Acute appendicitis occurs when luminal drainage is obstructed. Inflamed appendiceal mucosa secretes fluid which accumulates within the lumen, displacing the normally present gas and progressively distending the appendix. Edema and inflammation within the appendiceal wall result in wall thickening which also contributes to an increased total appendiceal diameter.
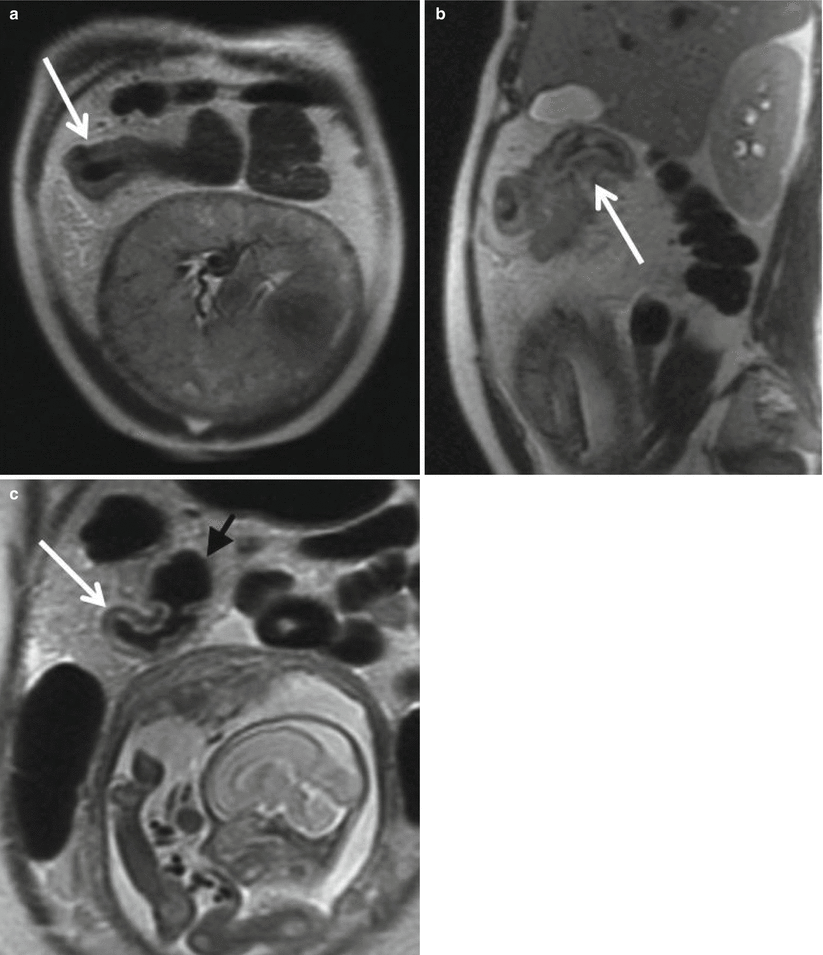
On MRI, an acutely inflamed appendix is often filled with hyperintense fluid on T2-weighted imaging, with an appendiceal diameter of 7 mm or greater considered abnormal (Fig. 23.3). The edematous wall of the appendix and sometimes the cecal tip appear brighter on T2-weighted images than the bowel wall elsewhere (Fig. 23.4) [26]. On diffusion-weighted images, there may be restricted diffusion of an inflamed appendix [2], increasing the sensitivity for the detection of acute appendicitis (Fig. 23.5) [27]. An appendicolith may be present, appearing as a hypointense focus on all imaging sequences, but not reliably seen by MRI (Fig. 23.6).
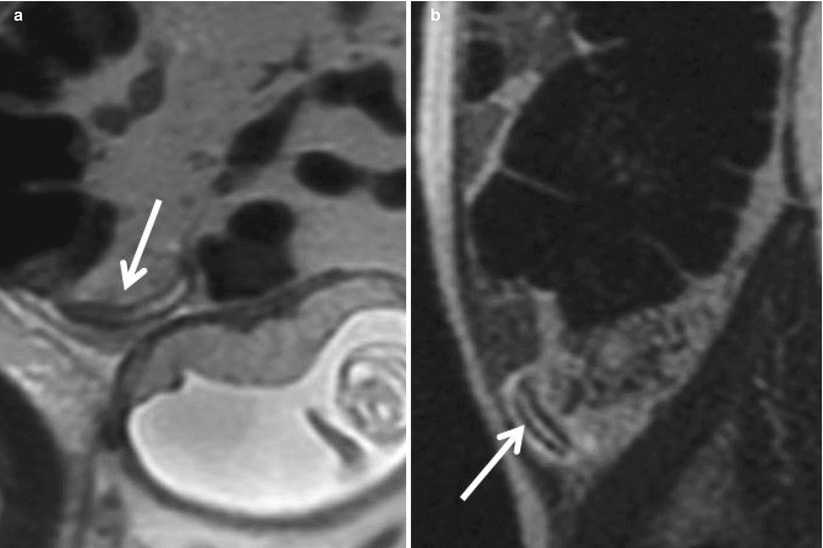
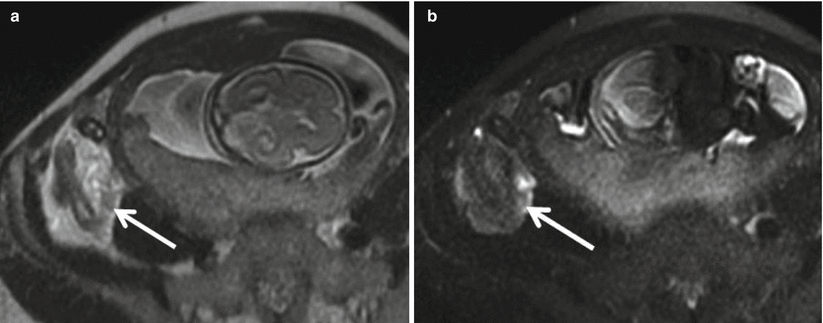
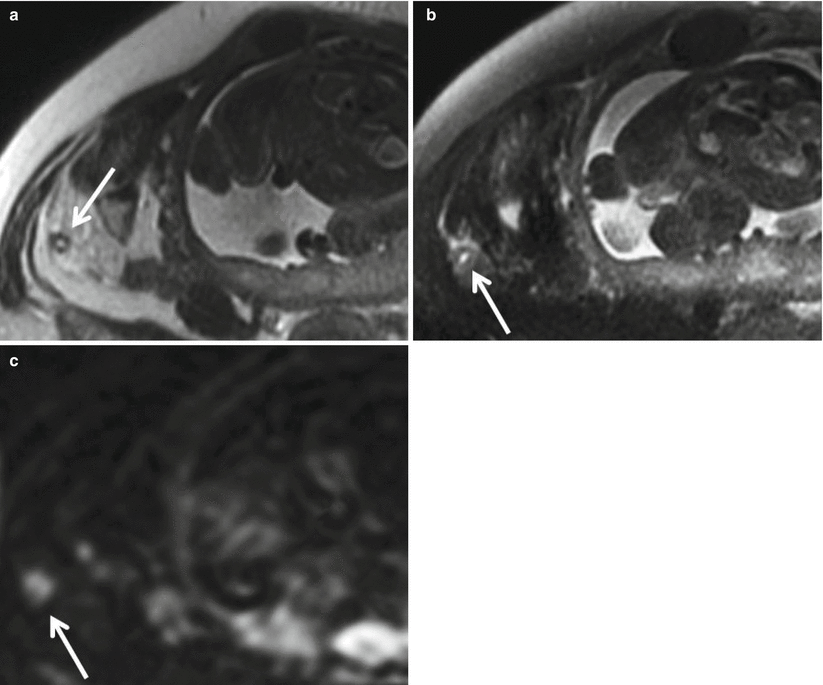
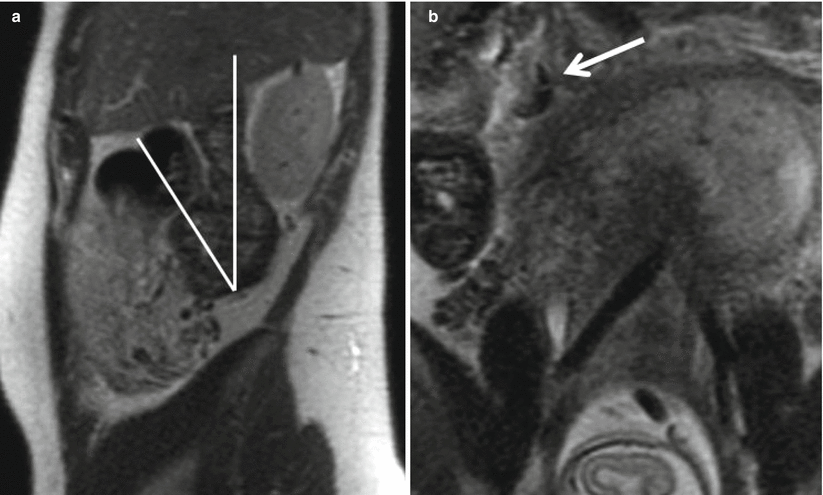

Fig. 23.3
Coronal (a) and sagittal (b) single-shot FSE images in a 28-year-old female at 16-week gestation demonstrate a fluid-filled, dilated appendix (arrow) with thickened walls and adjacent fat stranding compatible with acute appendicitis

Fig. 23.4
Axial single-shot T2-weighted images without (a) and with (b) fat suppression in a 34-year-old female at 23-week gestation demonstrate a dilated appendix with thickened and edematous wall (arrow) with adjacent edema and fat stranding confirming acute appendicitis

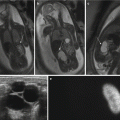
Fig. 23.5
(a) Axial T2-weighted FSE images without and (b) with fat suppression of a 41-year-old female pregnant with twins at 29-week gestational age demonstrate a thick-walled appendix (arrow) with mural edema, fluid within the lumen, and adjacent periappendiceal fat stranding compatible with acute appendicitis. (c) Axial DWI (b = 800 s/mm2) shows markedly high signal within the appendix compatible with restricted diffusion (arrow)

Fig. 23.6
(a) Sagittal single-shot FSE T2-WI demonstrating upward cecal displacement in a 21-year-old woman at 24-week gestational age with cecal tilt of approximately 140°. This angulation assisted in identifying the inflamed appendix within the right upper quadrant in this patient, with hypointense appendicoliths in the appendiceal base (arrow) on the (b) coronal single-shot FSE T2WI
Periappendiceal inflammation is indicated by stranding within the adjacent fat, appearing hyperintense on T2-weighted images which is particularly accentuated with fat suppression (Figs. 23.3, 23.4, and 23.5). While inflammation surrounding the appendix is usually a reliable sign of acute appendicitis, a small amount of free fluid is often seen within the right paracolic gutter during normal pregnancy, and this finding should be interpreted cautiously and in the context of other findings.
When the imaging appearance and clinical picture for appendicitis are equivocal, the risk of appendiceal perforation is likely low, and consideration may be given to close monitoring and potentially repeat imaging. If the appendix remains inflamed and not responsive to antibiotic therapy, subsequent imaging may show more definitive, evolved findings of appendicitis.
23.3.3 Perforated Appendicitis
Perforation is most frequently seen in the third trimester and is associated with marked negative effect on fetal outcome, including premature delivery. Unfortunately, the accuracy of all imaging modalities, including MRI, in identifying microperforation remains poor, with up to half of all cases of perforated appendicitis being misdiagnosed as simple appendicitis both by MRI and ultrasound [28]. Diagnosis of perforation can be suggested when the appendiceal wall appears discontinuous with a large amount of surrounding periappendiceal inflammation and fluid (Fig. 23.7). Confident diagnosis of perforation can be made when extraluminal gas or a periappendiceal fluid collection is identified. Diffusion-weighted imaging shows promise in prospective diagnosis of perforation when these definitive findings are absent. The degree of diffusion restriction has been shown to parallel increasing inflammation, with perforated appendices demonstrating significantly lower ADC values (0.79 mm2/s) than simple appendicitis (1.11 mm2/s) and normal appendices (1.85 mm2/s) (Fig. 23.8) [2].
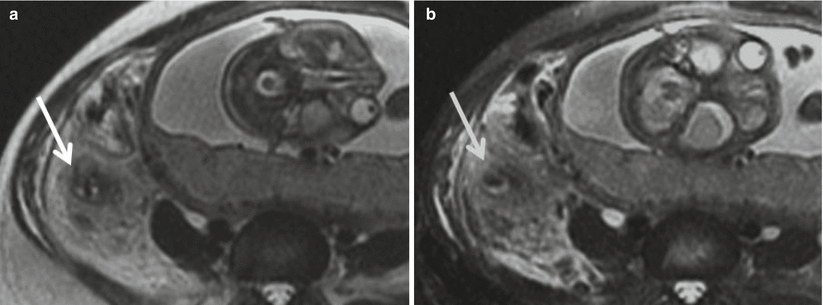
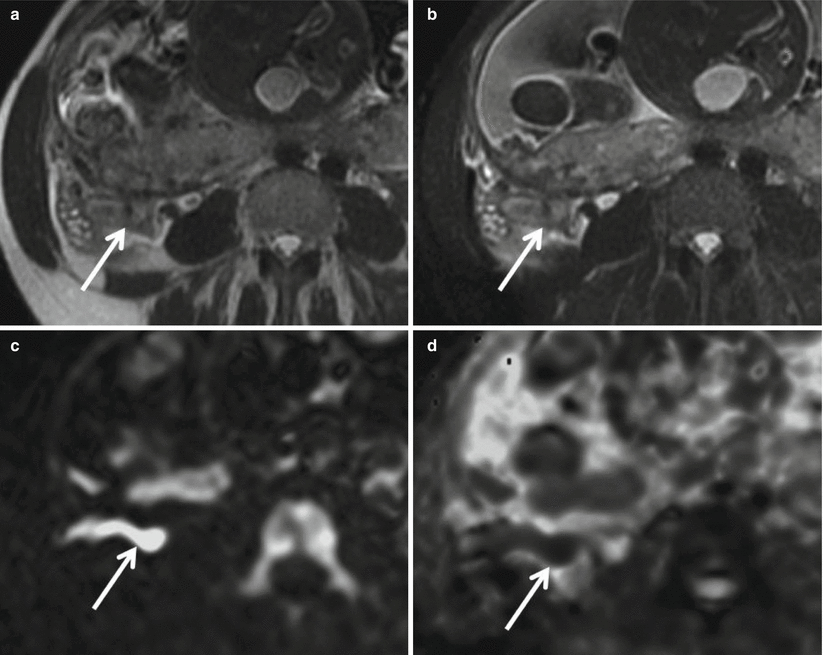

Fig. 23.7
(a) Axial, single-shot FSE T2-weighted image in this 36-year-old, 29-week pregnant woman demonstrates extensive fluid and edema surrounding an inflamed appendix, findings which are more readily apparent with (b) fat suppression which also highlights an irregular, discontinuous appendiceal wall (arrow). Although no discrete extraluminal fluid collection was identified, findings were highly suggestive of perforated appendicitis. Intraoperative and histopathologic evaluation confirmed acute gangrenous appendicitis with perforation

Fig. 23.8
Axial single-shot FSE T2-weighted images (a) without and (b) with fat suppression in a 32-year-old woman at 30 weeks of gestation demonstrate a dilated appendix with a fluid-filled lumen, mural thickening, edema, and periappendiceal stranding (arrow). Corresponding axial (c) DWI (b = 800 s/mm2) and (d) ADC map demonstrate hyperintense signal throughout the appendix on DWI and hypointensity on ADC with the cecal tip as having the greatest restriction of diffusion (ADC = 7.2 mm2/s) (arrow). Acute appendicitis with microperforation was confirmed surgically
23.3.4 Mimics of Appendicitis
Pitfalls in diagnosing appendicitis include misidentification and misinterpretation of the appendix. Many tubular structures within the right lower quadrant can be similar in caliber to the appendix. Identification of the cecal origin and blind-ending appendiceal tip allows for differentiation of the appendix from collapsed small bowel loops. Small vessels may be mistaken for a normal appendix, while engorged vessels of pregnancy, if confused for the appendix, may result in a false-positive diagnosis of appendicitis. The 2D time-of-flight (TOF) sequence is essential for differentiating the appendix from right lower quadrant vessels, such as the gonadal vein and pelvic varices. TOF images will depict blood flowing within vessels as high signal and the appendix with stationary contents as low signal (Fig. 23.9).
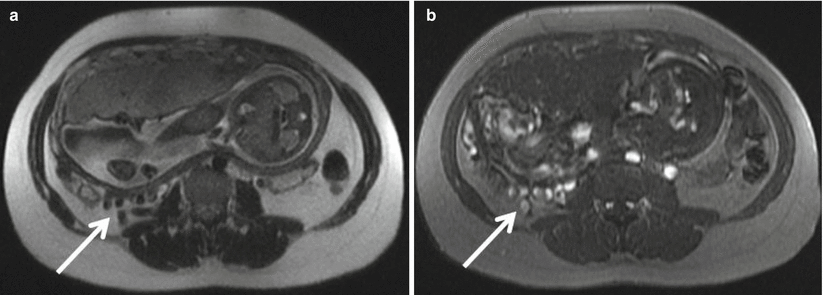

Fig. 23.9
(a) Axial single-shot FSE T2-weighted image in a 27-year-old patient at 26-weeks gestation shows a cluster of round and tubular structures in the posterior right lower quadrant, each a candidate for the appendix (arrow). (b) 2D TOF GRE image at the same level demonstrates hyperintense signal within these structures indicative of flow confirming that these structures are all vessels (arrow)
Chronic inflammation of the appendix may also present as mural thickening of the appendix and mimic acute appendicitis (Fig. 23.10). The lack of appendiceal luminal distention with fluid, mural edema, and adjacent periappendiceal inflammation along with the identification of intramural fat on chemical shift T1-weighted or fat-suppressed sequences within the appendix can help distinguish between acute and chronic inflammation of the appendix.
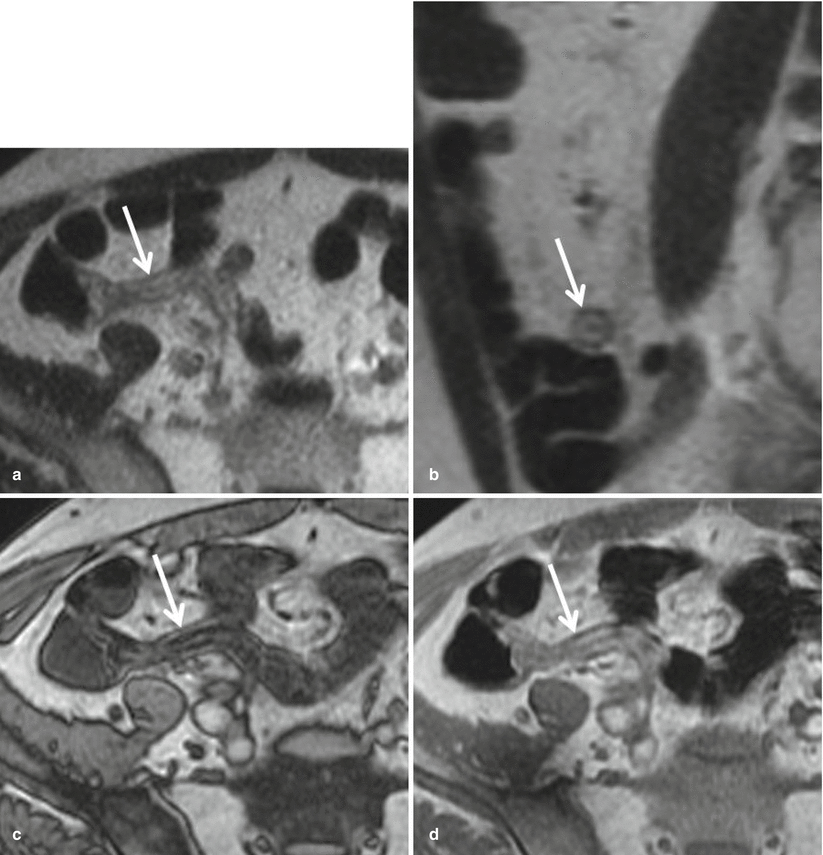

Fig. 23.10
(a) Axial and (b) sagittal single-shot FSE T2-weighted images demonstrate a thickened, hyperintense appendiceal wall. Note that the lumen is collapsed. While this may be mistaken for mural edema, chemical shift imaging confirms that the bright signal on T2WI is intraluminal fat, denoted by loss of signal in the appendiceal wall on (c) out-of-phase GRE T1WI compared to the (d) in-phase sequence (arrow). Presence of intramural fat can be seen due to the sequela of prior or chronic inflammation
23.3.4.1 Appendiceal Endometriosis
Endometriosis, a condition where ectopic endometrial tissue implants outside of the uterus, is a common disease and has potential for widespread involvement, including sites distant from the uterus. The appendix is involved in 2.8–4.1 % of cases of endometriosis and in 0.4 % of the general population [29, 30]. While usually asymptomatic, endometriosis may result in vague abdominal discomfort, and catamenial appendicitis has been speculated as cause of appendiceal pathology, thought to account for twice the frequency of appendectomies with no histologic findings of acute obstructive appendicitis in reproductive-aged women, as compared to age-matched males [31, 32]. True acute appendicitis secondary to endometriosis involvement can also result and should be considered as a cause for right lower quadrant pain in young women, particularly with known endometriosis [33]. Rarely, 0.03–0.08 % of pregnancies have been reported to be complicated by appendiceal endometriosis [34]. Endometrial implant growth responsive to hormonal influences of pregnancy may be responsible for the greater risk of perforation in these patients during the first two trimesters, with a hypothesized correlation between the degree of mural involvement of endometriosis and risk of perforation [35–37].
Soft tissue implants along the serosal surface of the appendix, typically hypointense to myometrium on T2- weighted images and isointense on T1-weighted images, may be seen in the setting of endometriosis. Cystic foci of hyperintensity on T2WI or hemorrhagic foci depicted by hyperintensity on T1WI are seen in a minority of cases (Fig. 23.11) [38]. Endometriosis implants can be characterized as superficial in one-third of cases and deep invasive in two-thirds of cases [39]. With invasion into the muscularis layer, secondary muscle hypertrophy, fibrosis, and retraction can be seen.
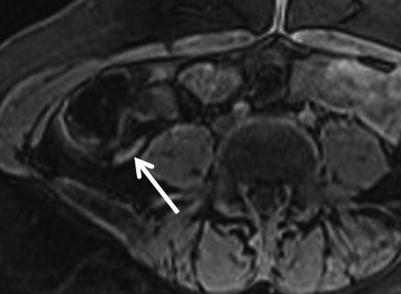

Fig. 23.11
Axial 3D fat-saturated spoiled GRE T1-weighted images of a woman presenting with right lower quadrant pain demonstrates high signal within the appendix compatible with endometriosis involvement of the appendix (arrow), which was confirmed surgically
Chronic, potentially cyclically active endometriosis of the appendix may also produce alternative appendiceal changes such as mucocele, periappendiceal inflammatory mass, intussusception, or ischemia [37].
Certainly, awareness of the potential for bowel involvement of endometriosis elsewhere besides the appendix is necessary. Bowel endometriosis is the most common site of extragenital involvement. The most common segment of the bowel involved is the rectum (52–65.7 %), followed by the sigmoid (17.4–19.4 %), the ileum (4.1–16.9 %), the appendix (5–6.4%), and the cecum (4.7–6.2 %) (Fig. 23.12) [38].
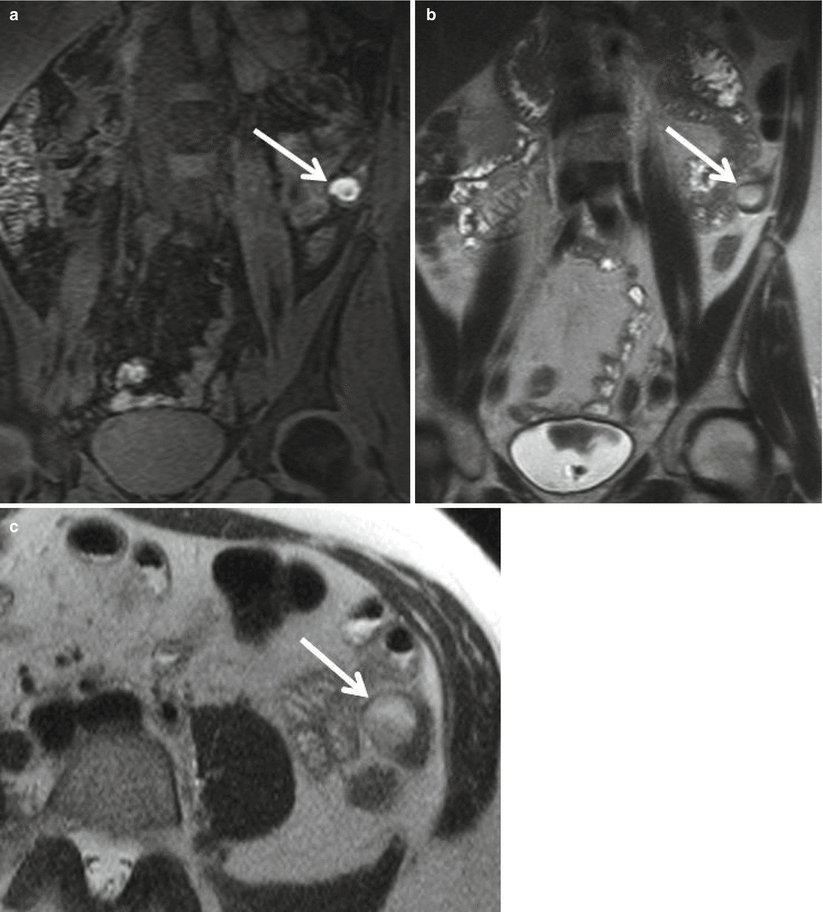

Fig. 23.12
(a) Coronal 3D fat-saturated spoiled GRE T1-weighted, (b) coronal, and (c) axial single-shot FSE T2-weighted images demonstrate an endometrial implant with associated endometrioma along the serosal surface of the descending colon (arrow). The implant demonstrates classic features of hyperintensity on T1WI, with shading on T2WI within the cystic component, denoting chronic blood products
23.3.4.2 Mucocele
Dilation of the appendix by intraluminal accumulation of mucin is an infrequent event, accounting for only approximately 0.3 % of appendiceal pathology [40]. Presentation is most often acute or chronic right lower quadrant pain. Etiologies can be classified into four histological groups ranging from benign to malignant entities: simple mucocele (20 %) which is a post-obstructive mucous retention cyst, mucosal hyperplasia (20 %), mucinous cystadenoma (50 %), and mucinous cystadenocarcinoma (10 %) [41].
Given depth of mural involvement, mucinous cystadenocarcinoma is the most frequent cause of mucocele to be complicated by pseudomyxoma peritonei; however, all histologic subtypes may result in pseudomyxoma peritonei, particularly with perforation [41, 42]. This complexity has resulted in much controversy and variability in the classification of mucoceles histologically [43]. Numerous classification schemes have been proposed attempting to accurately predict prognosis [41]. Consequently, preoperative diagnosis of appendiceal mucocele is crucial. When suspected preoperatively, resection with laparotomy is preferred over laparoscopy to minimize the risk of iatrogenic perforation and rupture [40].
On MRI, an appendiceal mucocele is a well-defined cystic structure, arising from the cecal tip, typically within the right iliac fossa, but can be variable in location during pregnancy. Extent of appendiceal distention usually correlates with underlying cause, with simple mucoceles and mucosal hyperplasia typically not exceeding 2 cm in diameter. Mucinous cystadenomas and cystadenocarcinomas can reach up to 6 cm in diameter [41]. The contents of a mucocele on MRI are usually homogeneously high in signal intensity on T2-weighted images (Fig. 23.13). High protein content results in variable signal intensity on T1-weighted images, but typically intermediate [44]. Unlike acute appendicitis, mucoceles typically lack periappendiceal stranding and mural thickening unless complicated by superimposed inflammation or rupture [45]. Presence of nodularity should raise suspicion for a mucinous cystadenocarcinoma, and internal vascularity can be further assessed with ultrasound as enhancement is typically not assessed in the setting of pregnancy. Correlation with features from other imaging modalities such as curvilinear mural calcifications on CT and concentric “onion skin” appearance on ultrasound may increase the specificity for the diagnosis of a mucocele. When perforated, the MR imaging features of ruptured mucocele are virtually indistinguishable from perforated acute appendicitis [46].
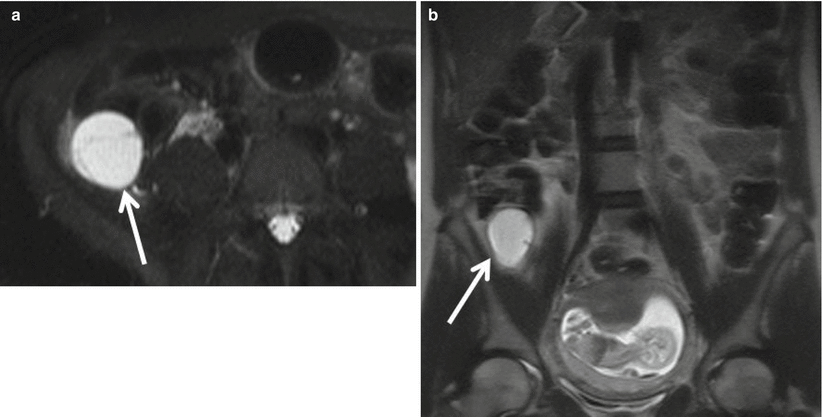

Fig. 23.13
(a) Axial single-shot T2WI with fat suppression and (b) coronal single-shot FSE T2WI in a 36-year-old, 14-week pregnant patient demonstrating a markedly distended appendix with hyperintense material (arrow). The degree of appendiceal distension without associated wall thickening or periappendiceal inflammation is highly suggestive of a mucocele. Pathology confirmed mucinous cystadenoma without carcinoma
Mucocele of the appendix has been shown to have an association with additional malignancies. The incidence of colorectal cancer in this population is approximately six times greater than the general population, approximately 17 % [41]. Breast, ovarian, and renal tumors have also been observed at a higher frequency in patients with appendiceal mucoceles [47, 48]. Awareness of these associations is critical for appropriate subsequent screening.
23.4 Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) as the cause of abdominal pain in the pregnant population should be considered in both those women already carrying the diagnosis and women not previously diagnosed, as the childbearing years coincide with the peak age of onset of both Crohn disease and ulcerative colitis [49]. There are known effects of pregnancy on the activity of IBD. Historically, it has been believed that overall IBD activity during pregnancy approximates that of nonpregnant patients. The activity of disease at conception is used as a predictor of activity during pregnancy: when quiescent at conception, about a third of patients are expected to experience relapse, while two-thirds of patients with active disease at conception are expected to continue to have active disease during pregnancy [50, 51]. More recently, it has been elucidated that, indeed, no statistically significant difference in the course of Crohn disease is expected in pregnancy as compared to nonpregnant women, confirming no effect of pregnancy on Crohn disease. Patients with ulcerative colitis, however, have a greater than twice risk of relapse during pregnancy as compared to their nonpregnant counterparts, particularly during the first and second trimesters, as well as during the first 6 months postpartum. The pathophysiology of this progression may relate to smoking cessation or placental cytokine secretion, although it is not entirely clear [52]. Particular attention has been paid to the effects of vaginal delivery on perianal disease. While the trend and recommendation is to avoid vaginal delivery with active perianal disease, there has been no evidence of vaginal deliveries inciting development or exacerbation of perianal Crohn disease or protective effect of Cesarean section in progression of disease. In the absence of active perianal disease, Crohn disease patients can follow general population guidelines for mode of delivery, but episiotomy should be avoided [53–55].
Effects of IBD on pregnancy have also been extensively studied. The degree of IBD activity at conception and throughout gestation correlates with pregnancy outcome, with only active IBD increasing the risk for poor fetal outcomes, including preterm birth, low birth weight, and fetal loss [55, 56].
23.4.1 Crohn Disease
Given the potential significant morbidity to both mother and fetus in active Crohn disease, prompt and accurate diagnosis is necessary to initiate management and plan surgical intervention when necessary. While endoscopic evaluation of the colon and distal ileum can be performed, cross-sectional imaging often is necessary for small bowel assessment and identification of penetrating complications [57].
MR enterography (MRE) has long been established as a safe and accurate method of assessing Crohn disease [58]. Conventional MRE evaluation for bowel inflammation relies on intravenous contrast use for assessment of enhancement as a marker of vascularity and perfusion [59]. The combination of extent and pattern of bowel enhancement, together with other MRI features such as wall thickness, edema, and mucosal ulcerations, has been shown to correlate with endoscopic findings and the widely used Crohn Disease Endoscopic Index of Severity [60]. Subsequently, a simpler scoring system using only mural thickness and signal of the bowel wall on T2-weighted sequences has been shown to correlate with both the MRI Activity Index and a histopathological score of terminal ileitis [61]. In the general population, MRE has been shown to have a sensitivity of 91 % and specificity of 71 % for the identification of active inflammatory bowel disease [62].
In the setting of pregnancy, there are several limitations which may result in a less than optimal MR imaging evaluation of the maternal bowel. Motion degradation is a frequent difficulty encountered with imaging the pregnant patient. This is due to a combination of multiple moving parts (fetus, amniotic fluid, maternal bowel, and maternal breathing) coupled with relatively long acquisition times for the individual sequences. Additionally, intramuscular glucagon, an antiperistaltic agent typically used with MRE in nonpregnant patients to help decrease bowel motion, is considered a pregnancy class C drug by the FDA and usually should be avoided as it could potentially have adverse effects upon the fetus. The avoidance of intravenous gadolinium-based contrast use in pregnancy also further hampers the assessment of Crohn disease on MRI.
These limitations can partly be overcome by the use of fast, motion-insensitive sequences and respiratory-triggering techniques. Furthermore, the administration of inert oral contrast agents such as dilute barium (VoLumen; E-Z-EM, Bracco Diagnostics, Monroe Township, NJ) which have no known detrimental effects on the fetus can potentially be helpful in the distention and imaging assessment of the small bowel. Approximately 900 ml of such an oral agent is recommended followed by 500 mL of water to the best of the patient’s ability and tolerance.
Despite these limitations, MRE has been shown to play a key role in the management of pregnant patients with Crohn disease. Findings depicted on MRE not only can stratify patients into those necessitating emergent surgical management and those in whom interventional procedures for Crohn disease can be delayed until postpartum, but also aid in delivery decisions [57].
23.4.1.1 Crohn Disease Imaging Features
Crohn disease can involve any segment of the bowel in a continuous or skip fashion, but most frequently involves the terminal ileum.
Uncomplicated active Crohn disease can be identified as a segment of circumferentially thickened, edematous bowel wall (Fig. 23.14). Wall edema results in hyperintense signal on T2-weighted images, particularly well visualized on fat-suppressed sequences. Mural stratification on T2-weighted images where a central layer of hyperintense signal is flanked by intermediate signal intensity within the bowel wall is often a feature seen in active inflammation [63]. Free fluid and edema in surrounding soft tissues are indicative of a transmural inflammatory process. Luminal narrowing is seen both with active disease (Fig. 23.14) and the sequela of chronic inflammation with secondary stricturing and fibrosis (Fig. 23.15). The latter may be identified by the lack of associated mural edema and adjacent fat stranding, as well as dilation or obstruction of upstream bowel. Dynamic multiphase, multislice, cine T2-weighted imaging is useful in assessing bowel peristalsis and distensibility, with diseased, inflamed, or fibrotic segments typically being relatively adynamic as compared to uninvolved loops of bowel.