Fig. 1.
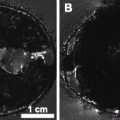
3D high-resolution whole body MRI of fixed mouse embryos (16.5 days of development). Multiple embryos in glass container, FOV (25.6 mm)3 with an isotropic resolution of 50 μm. (a) Axial slices demonstrating the four embryos (transverse sections). The embryos are embedded in 1% agarose (AG) with gadolinium and separated by a crossed plastic partition (P). (b) Sagittal slice through one of the embryos. (c) Detail of the heart (transverse section) of the embryo in b. B, brain; H, heart; I, intestine; Li, liver; Lu, lung; LV, left ventricle; RV, right ventricle; T, tongue; V, vertebrae.
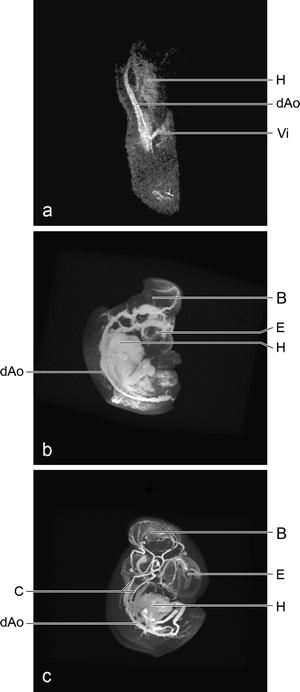
Fig. 2.
Contrast-enhanced cardiovascular system of fixed chicken embryos, maximum intensity projections. (a) The vasculature of this young embryo of only 2.5 days of development consists of a looped heart (H) and a pair of dorsal aortae (dAo) leading to the vitelline (Vi) blood vessels. FOV 8 × 4 × 4 mm, isotropic resolution of 31 μm. (b) With 4 days of development the vasculature has developed considerably. Besides the blood vessels in the thorax and abdomen, those of the brain (B) and eyes (E) are demonstrated. FOV (10 mm)3, isotropic resolution of 39 μm. (c) The final configuration of the major branches are developed at 5 days of development. FOV (14 mm)3, isotropic resolution of 55 μm. C, carotid arteries.
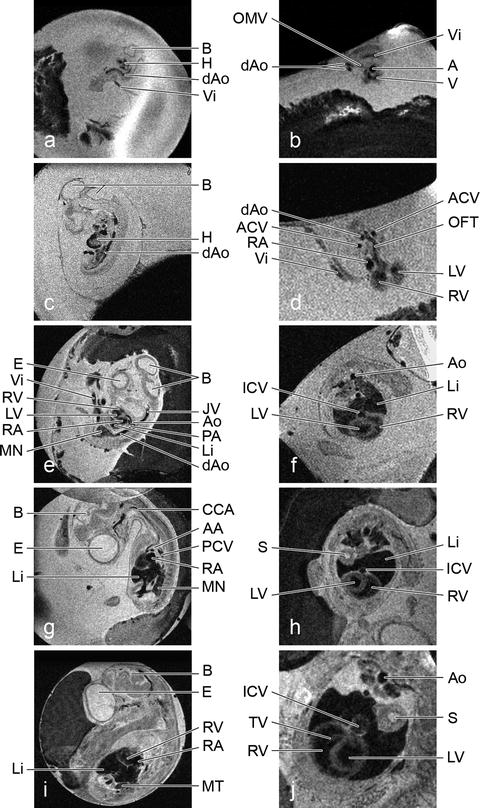
Fig. 3.
In ovo time course imaging of quail development. Left-hand images are sagittal slices (0.5 mm) through the same quail embryo at successive embryonic days (ED) 3 (a), 5 (c), 7 (e), 9 (g) and 11 (i) with FOVs of (25.6 mm)2 and a resolutions of 100 × 100 μm2 while the right-hand images are the transverse sections (0.3 mm) through these hearts ED 3 (b), 5 (d), 7 (f), 9 (h), 11 (j) with FOVs of (22 mm)2 and resolutions of 86 × 86 μm2. A, atrium; AA, aortic arch; ACV, anterior cardinal vein; Ao, aorta; B, brain; CCA, common carotid artery; dAo, dorsal aorta; E, eye; H, heart; ICV, inferior caval vein; JV, jugular vein; Li, liver; LV, left ventricle; MN, mesonephros; MT, metanephros; OFT, outflow tract; OMV, omphalomesenteric vein; PA, pulmonary artery; PCV, posterior cardinal vein; RA, right atrium; RV, right ventricle; S, stomach; TV, tricuspid valve; V, ventricle; Vi, vitelline vessel.
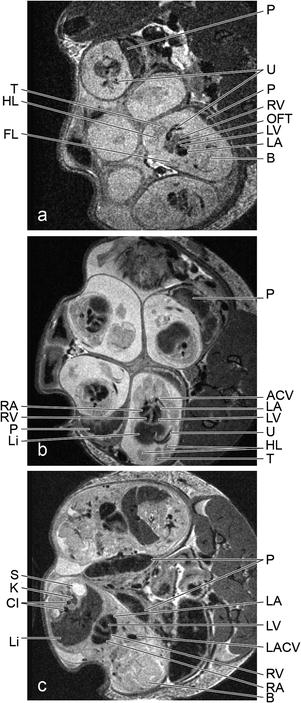
Fig. 4.
In utero time course imaging of mouse development. Non-triggered axial slices (0.5 mm) through the abdomen of a pregnant dam at ED 12.5 (a), 14.5 (b) and 17.5 (c) with FOVs of (25.6 mm)2 and resolutions of 100 × 100 μm2. ACV, anterior cardinal vein; B, brain; CI, common iliac arteries; FL, forelimb; HL, hind limb; K, kidney; LA, left atrium; LACV, left anterior cardinal vein; Li, liver; LV, left ventricle; OFT, outflow tract; P, placenta; RA, right atrium; RV, right ventricle; S, stomach; T, tail; U, umbilical vessels.
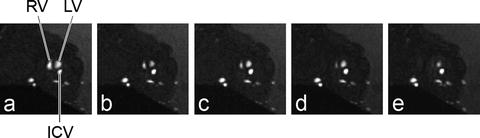
Fig. 5.
In ovo cardiac imaging. Retrospectively gated (IntraAngio) movie frames of one transverse slice (0.3 mm) through an embryonic quail heart of 9 days of development with a FOV of (22 mm)2 and a resolution of 172 μm. Frame 2/10 (a), 4/10 (b), 6/10 (c), 8/10 (d) and 10/10 (e). The different phases of the cardiac cycle are clearly visible, going from diastole (a) with completely filled right (RV) and left ventricles (LV) to systole (e) with almost empty ventricles. ICV, inferior caval vein.
2 Materials
2.1 Embryos In Vitro
1.
Embryos of the desired stage of development
a.
Fertilized White Leghorn (Gallus gallus domesticus) or quail (Coturnix coturnix japonica) eggs incubated at 37°C and 60–70% relative humidity.
b.
Mouse or rat embryos excised from the uterus.
2.
Rinsing and preparation fluid: Phosphate-buffered saline (PBS). Store at room temperature, use at 37°.
3.
Glass containers, matching the size of the embryos.
2.1.1 Whole Body
1.
Prepare 8% paraformaldehyde (500 mL): Place a conical flask with 400 mL purified water on top of a hot plate/stirrer inside a fume hood. Set the heater on and use moderate stirring. Slowly add 40 g paraformaldehyde (see Note 1). Add 10 drops of 1 M NaOH after a few minutes. The solution will turn from cloudy to clear when ready. Switch off the heat but keep on stirring while cooling down. When cooled, fill up solution to 500 mL with purified water and filter when necessary. Store at 4°C.
2.
Prepare 0.2 M phosphate buffer (500 mL): add 5.52 g NaH2PO4·1H2O to 200 mL purified water (= 0.2 M base). Add 10.78 g Na2HPO4·2H2O to 300 mL purified water (= 0.2 M acid). Add 0.2 M acid into the flask with 0.2 M base until pH is 7.4. Store at 4°C.
3.
Prepare fresh 4% paraformaldehyde fixative (4%PFA) by adding equal amounts of 8%PFA to 0.2 M phosphate buffer.
4.
Prepare fixative with gadolinium contrast agent: add 200 μL Dotarem (Guerbet, Paris, France) to 10 ml 4%PFA.
5.
Embedding: 1% agarose LE Analytical Grade, dissolved in PBS, supplemented with 1:50 v/v Dotarem.
2.1.2 Cardiovascular System
1.
PBS. Store at room temperature and heat before injection to 37°C.
2.
Gadolinium-DOTA, 0.5 mM (Dotarem; Guerbet, Paris, France).
3.
Gelatin (Merck, Darmstadt, Germany).
4.
Horizontal pipette puller (Bachofer GmbH, Reutlingen, Germany).
5.
Capillaries (2.0 mm O.D., 1.16 mm I.D.) (Clark Electromedical Instruments, Pangbourne, UK).
6.
Disposable needles (21G) and disposable syringes (1 mL).
7.
Dissecting microscope with a micromanipulator.
8.
For young embryos: Petri dishes half-filled with 5% agarose.
9.
Perfluoropolyether, e.g. Fomblin (FenS Chemicals, Goes, the Netherlands).
10.
Prepare glass needles: First pull glass needles with tip diameters 4–20 μm from capillaries. Mount them on disposable injection needles (21G) with two-component glue (local hardware store) and connect each needle to a disposable 1 mL syringe.
2.2 Embryos In Ovo
1.
Incubator installed in the scanner room.
2.
Fertilized White Leghorn (Gallus gallus domesticus) or quail (Coturnix coturnix japonica) eggs incubated at 37°C and 60–70% relative humidity.
3.
For embryos ≥ ED10: Ketanest-S, 5 mg/mL S-ketamine (Pfizer, Capelle, the Netherlands).
4.
Disposable syringes, needles and tape.
2.3 Embryos In Utero
1.
Pregnant mouse dam.
2.
Respiration monitoring system (SA Instruments, Stony Brook, NY, USA).
3.
Isoflurane inhalation equipment (UNO Roestvaststaal, Zevenaar, the Netherlands), including medical oxygen, medical air and isoflurane (PCH Pharmachemie, Haarlem, the Netherlands).
2.4 MRI
The MRI sequences provided here are based on a vertical 9.4 T micro-imaging system (Bruker Biospin, Rheinstetten, Germany) with an actively shielded Micro2.5 gradient (1 T/m, rise time < 110 μs), a BOS-II shim system, pre-emphasis and B 0 compensation. ParaVision 4.0 was used for image acquisition and analysis.
3 Methods
3.1 Embryos In Vitro
1.
Remove embryo from egg or uterus and transfer it to a Petri dish filled with PBS (see Note 2).
2.
Carefully remove all membranes.
3.1.1 Whole Body
1.
Fix embryos overnight by immersion (see Note 3).
2.
Prepare embedding solution: place a conical flask with 50 mL PBS on top of a hotplate/stirrer. Switch the heater on and use fast stirring. Add 1 mL Dotarem followed by 0.5 g agarose. Keep on heating until the solution is clear.
3.




When multiple embryos are scanned simultaneously, take care to mark individual embryos, e.g. by removing (parts of) a right or left extremity. In addition, small plastic or glass rods can be inserted next to a particular embryo.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree