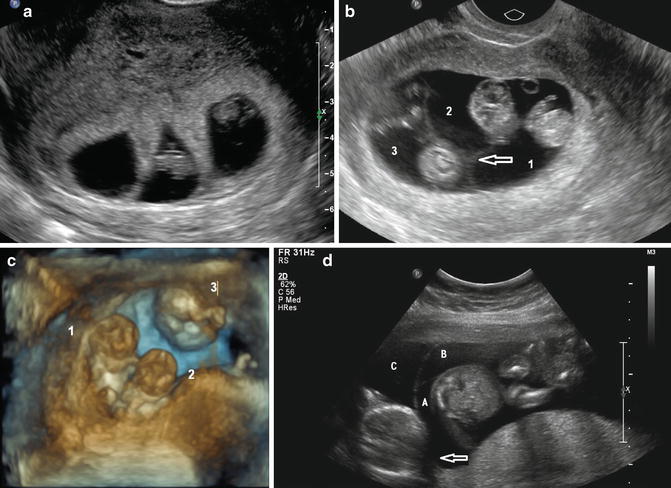
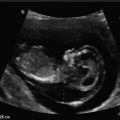
Fig. 14.1
Multiple corpora lutei. This is indicative of multiple follicular ovulation. Multiple corpora lutei can be a sign of a dizygotic pregnancy; however, it is also frequently seen with the use of fertility-enhancing medications
Embryological development of the fetus is addressed in details in Chap. 2 of this book. Multiple gestations can be the result of a single oocyte being fertilized by a single spermatozoon with splitting of the resulting zygote at various times (monozygotic [MZ] twins) or multiple oocytes (two or more), each fertilized by its own spermatozoon, resulting in two or more zygotes (dizygotic twins [DZ], or higher degree multiples). Dizygotic twins are more common (70 %), and are also known as “fraternal twins” since, genetically, the two zygotes that resulted are as different as two regular siblings (e.g., opposite genders). The incidence of DZ twins increases with maternal age, parity, ovulation induction and they are more common in some families, with mothers of DZ twins reporting significantly more female family members with DZ twins than mothers of monozygotic twins. Maternal factors such as genetic history, advanced age, and increased parity are known to increase the risk of DZ twins [35]. New findings indicate some women may have a genetic predilection to conceive twins, specifically insertion/deletions and missense alterations in the growth differentiation factor 9 (GDF9) sequence in mothers of twins [36, 37]. Rates of DZ twins have a geographical variation with some countries/continents such as South and South East Asia as well as Latin America exhibiting low prevalence, e.g., 6–9 twin sets per thousand births [38], and rates being much more common in some ethnicities, such as in Nigeria, where the Yoruba have the highest rate of twinning in the world, at 45–50 twin sets per 1000 live births, possibly due to high consumption of a specific type of yam containing a natural phytoestrogen [39]. Dizygotic twins will always be dichorionic–diamniotic (DCDA). Monozygotic twins are known as “identical twins” since they originate from a single zygote and are thus genetically identical (with exceptions, see below). They comprise 30 % of twins and their incidence is sporadic, with no family predilection and with a rate similar throughout the world (1:250 pregnancies). In MZ twins, the time of splitting will determine placentation, chorionicity and amnionicity (see below, placentation). The prevalence of females compared to males increases progressively from a relatively equal prevalence in singletons to a clear preponderance in conjoined twins.
Diagnosis
Before the development of ultrasound, twins were often diagnosed at birth, after the delivery of one neonate. In fact, a multiple gestation was clinically suspected in only 25–50 %. In the famous Routine Antenatal Diagnostic Imaging with Ultrasound Study (RADIUS), 38 % of twins were recognized after 26 weeks and 13 % were not diagnosed until delivery [40]. In the Helsinki Ultrasound Trial, 25 % twins were not recognized until 21 weeks [41]. These two studies, however, were not really about first trimester ultrasound but rather about scanning at mid-trimester (16–24 weeks). The diagnosis should be obtainable, with ultrasound, from very early in gestation. When ultrasound is performed for an indication (e.g., the uterus is larger than expected), the accuracy is about 75 %. When ultrasound is performed routinely, this climbs to 90 % [42], with better outcomes in women known to carry multiple gestations [43]. The first ultrasound indication of a multiple gestation may be the presence of multiple corpora lutei (see Fig. 14.1). While routine ultrasound is still not the official rule, as recommended in low risk pregnancies by the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR) or the American Institute of Ultrasound in Medicine (AIUM), the advantages of a policy of routine scanning in the first trimester include, among others, the early detection of multiple gestations, allowing for early determination of chorionicity and amnionicity [44, 45]. Another clear advantage is accurate assessment of gestational age (GA). When ultrasound is ordered “to date” the pregnancy, in cases of unknown or unclear last menstrual period, fetal biometry is used to determine GA. In twins, however, there may be growth discordancy, for instance with one twin measuring 1 week more than the other. The published literature does not provide evidence-based data on whether dating should be based on the smaller twin, the larger or an average. It is important, however, to avoid missing early growth restriction in one twin, thus, the majority will date the pregnancy based on biometry of the larger twin [46]. An important consideration is whether growth nomograms for singleton gestations can be used for twins or higher order gestations [47]. It appears that during the first trimester, there are no major differences in fetal biometry between singleton and multiple pregnancies [46]. Hence, crown-rump-length (CRL) curves published for singletons may be used in the assessment of twins and triplets [48, 49]. Furthermore, there is no difference in placental mass between singletons, monochorionic (MC) and dichorionic (DC) twins and trichorionic triplets between 11 and 13 6/7 weeks [50]. Growth curves for singletons may be used in the assessment of biometry in twins until approximately 34 weeks GA [51]. For triplets, the upper limit may be lower, e.g., 25 weeks [52].
Placentation
Determining the number of chorionic sacs is important because prognosis is much better in DC than MC twin pregnancies [53]. Mortality (stillbirth, perinatal, and neonatal death) is 3–4 times higher in MC twins [53–58]. The major reason is the presence of vascular anastomoses between the two placental circulations [59–61]. They are at risk of twin-to-twin transfusion syndrome or TTTS [62–66], twin anemia–polycythemia syndrome or TAPS [67–69], twin reversed arterial perfusion or TRAP syndrome [70, 71], unequal placental sharing with discordant twin growth or selective intrauterine fetal growth restriction [72], and, if also monoamniotic (MA), cord entanglement with the added risk of demise of one twin and embolization of thromboplastin from the demised fetus to the healthy twin [73–75]. Additionally, there is the risk of conjoining, an event occurring in 1/50,000 births [76]. Mortality is 8–10 % in DCDA, 25 % in MCDA, 50–60 % in MCMA, and perhaps 90 % in conjoined twins [77–79] with fetal loss under 24 weeks, 1.8 % in DC twins, and 12 % in MC twins [53].
As described above, approximately 70 % of twins delivered and conceived naturally, result from the fertilization of two independent oocytes, i.e., dizygotic (DZ) twins; the remaining 30 % are the result of the division of a single zygote, i.e., monozygotic (MZ) twins. Interestingly, the rate of MZ twins is three times higher in pregnancies conceived with the help of ART, compared to spontaneous conceptions [80, 81]. If the division of the zygote occurs at the two-cells stage (0–4 days), before the morula stage, this results in two morulas, two blastocysts, two chorions, and two amnions (dichorionic–diamniotic or DCDA placentation), about one-third of monozygotic twins. In about two-thirds of monozygotic twins, the split occurs after the morula stage (4–7 days) and the single morula split will result in MCDA placentation. If it occurs at 7–14 days, the embryonic disc was already formed and the result will be two embryos in the same sac (MCMA). If after day 13–14, conjoined twins will result. A combination of both may also exist, when one of two dizygotic twins splits, in a monozygotic fashion, resulting in various combinations of chorionicity and amnionicity.
Ultrasound plays an important, if not the major, role in the determination of chorionicity and amnionicity early in pregnancy [44, 72, 82–94]. Various algorithms themes can be used, based on what is the known (or assumed) gestational age [95–97]. With appropriate training, reproducibility of the results has been shown to be excellent [98].
From 4 to 6 weeks, the number of sacs determine the chorionicity: two sacs means twins are DC (Fig. 14.2).


Fig. 14.2
Dichorionic diamniotic twins, 5 weeks. (a) In this retroverted uterus, two separate sacs are distinguished at 5 weeks. (b) 3D image a few days later demonstrates the presence of two fetal poles (arrows)
From 6 to 8 weeks, if the number of sacs is the same as the number of yolk sacs and the number of fetuses, this is a DCDA pregnancy. If the pregnancy is MC, two fetuses will be visualized within the sac and the number of yolk sacs will help distinguish between DA and MA placentation. Observing two yolk sacs or two clear amniotic cavities (Fig. 14.3) allows one to make the diagnosis of diamniotic twins [99]. Visualization of two fetal poles with a single yolk sac is diagnostic of monoamnionicity (Fig. 14.4). Once the membranes can be visualized, ultrasound imaging can distinguish between MC and DC twin pregnancies with more than 90 % accuracy [93]. The “twin peak,” also called lambda sign, at the level of the attachment of the chorionic membranes to the placenta is formed by projection of the trophoblast from a fused dichorionic placenta between the layers of the membranes and indicates a DC twin pregnancy (Fig. 14.5), with 100 % accuracy, while the “T sign” at the site where the thin inter-twin membrane composed of two amnions with no chorions leaves the placenta at a 90° angle (Fig. 14.6) indicates a monochorionic–diamniotic (MCDA) twin pregnancy [100]. In a study of 55 cases, sensitivity of the twin-peak sign for dichorionicity was 94 %, specificity 88 %, positive predictive value 97 %, and negative predictive value 78 % [101]. In another study of 506 DC and 154 MC twin pregnancies, between 11 and 14 weeks of gestation, use of the twin-peak and T signs and the number of placentas had sensitivity of 100 % specificity of 99.8 % for monochorionicity, with only one DC pregnancy incorrectly assigned as MC [102].





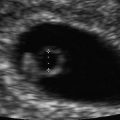
Fig. 14.3
Diamniotic twins. Two clearly separate amniotic cavities are distinguished. Amniotic membranes are marked by arrows

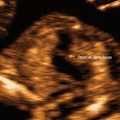
Fig. 14.4
Monoamniotic twins, 6 weeks. (a) Two fetal poles are visualized and only one yolk sac is demonstrated (arrow). (b) At 10 weeks, three-dimensional ultrasound demonstrates both twins in a single sac, with no intervening membrane

Fig. 14.5
Twin peak or lambda sign. Two-dimensional B-mode (a) and three-dimensional (b) ultrasound of a dichorionic diamniotic pregnancy. The white arrows point to where the “peak” is formed from the two placentas abutting. The inter-twin membrane (yellow arrow) appears thin. If the lambda sign was absent, accurately determination of chorionicity would be challenging

Fig. 14.6
T sign. The arrows point to a thin membrane, connecting to the placenta at a right angle, forming the letter T. This is diagnostic for a monochorionic placentation
From 8 to 14 weeks, the number of placental masses and/or the lambda or T sign can be assessed, as above, but at that stage, membrane thickness can also be analyzed [88]. A dichorionic membrane is typically well defined and easy to visualize with ultrasound. It consists of four layers (i.e., two layers of both amnion and chorion) and its width will be greater than 2 mm (Fig. 14.7). The presence of a thick dividing membrane indicated a dichorionic–diamniotic gestation in 38 (90 %) of 42 cases in which it was identified [103]. The number of dividing membranes can also, occasionally, be counted: four means DCDA (Fig. 14.8), two means MCDA [95].



Fig. 14.7
Dichorionic diamniotic membrane. (a) Membrane measures more than 2 mm in width. (b) Four layers (two chorionic and two amniotic membranes) can be visualized

Fig. 14.8
Monochorionic diamniotic membrane. The membrane is thin (1.4 mm) and elusive
For women presenting after 14 weeks 0 days, all of the above features should be used and, in addition, evaluation of fetal gender, since discordancy would, obviously, signify dizygocity [104]. In the second and third trimesters, membrane thickness is much less useful [105].
If transabdominal views are poor because of elevated BMI or retroverted uterus, transvaginal ultrasound is recommended. In a study by Bora and colleagues [106], chorionicity and amnionicity were documented in 67 viable twin pregnancies at both 7–9 and 11–14 weeks’ gestation. There was agreement in the chorionicity and amnionicity reported at each of the two scans in 65 out of 67 (97 %) cases. Of the DCDA pregnancies reported at 7–9 weeks, 53 out of 54 (98 %) were confirmed at the 11- to 14-week scan and one (2 %) was found to be MCDA. At birth, however, these twins were of different sex, confirming DCDA twins as initially diagnosed at 7–9 weeks. Of the 12 pregnancies diagnosed as MCDA at 7–9 weeks, all were found to be MCDA at the 11- to 14-week scan. In the (rare) case when chorionicity cannot be established, management should be based on the assumption that the gestation is monochorionic, until proved otherwise. After ultrasound diagnosis and characterization of twins, the risk of spontaneous loss of both fetuses before 22 weeks of gestation is significantly higher in MC than in DC pregnancies, and is significantly higher in MCMA pregnancies than in MCDA pregnancies [107]. Hence, no ultrasound report on twins should be considered finalized without details of the type of placentation [108]. Another sign has been described in triplet pregnancy: the ipsilon zone, the junction of the three interfetal membranes with 100 % success in determining chorionicity in 19 sets of triplets [109].
Another important role for ultrasound in multiple gestation is observation of the umbilical cord insertions in the placenta. Abnormal cord insertions such as marginal and velamentous insertions are much more frequent in multiple gestation (Fig. 14.9). In addition, single umbilical artery is also much more frequent in twins [110, 111].
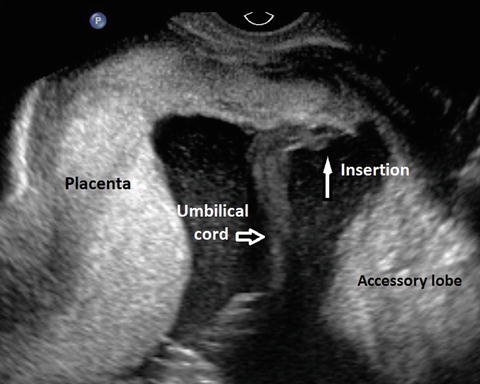

Fig. 14.9
Velamentous insertion of the cord in a twin pregnancy
Complications
Several complications are unique to multiple gestations: vanishing twin, death of one fetus, discordant fetal growth, discordance for genetic/structural anomaly, and partial mole. Some will only be found in monochorionic gestations (TTTS, TAPS, TRAP) while conjoined twins and cord entanglement are specific for MCMA gestations, which have been called “the most precarious of twin pregnancies” [112].
Vanishing Twin
This refers to a phenomenon, first described by ultrasound in 1982 [113], where, after documentation of multiple fetal heart activity, one embryo may not be visualized in a subsequent ultrasound. In fact, among gestations that start as twins, approximately one third will ultimately result in singletons and about 10 % will result in no fetuses. Multiple pregnancies may constitute more than 12 % of all natural conceptions, of which only about 2 % survive to term as twins and about 12 % result in single births [114]. In pregnancies diagnosed as twins prior to 7 weeks of gestation spontaneous reduction of one or more gestational sacs and/or embryos occurred before the twelfth week of gestation in 36 % of twin, 53 % of triplet, and 65 % of quadruplet pregnancies [115]. As evident from the above numbers, the phenomenon is even more common in higher order multiples [116], occurring in up to 50 % of triplet pregnancies with a triplets delivery rate of 47.4 % among 38 pregnancies diagnosed around 7 weeks with triplets, whereas 31.6 % delivered twins, 18.4 % delivered singletons, and only one patient miscarried all three cases [117]. The ultrasound diagnosis includes complete disappearance of a previously clearly demonstrated gestational sac and/or embryo or sonographic findings, indicating a failed pregnancy: sac smaller than expected, with irregular margins, crescent as opposed to sphere shaped or incomplete trophoblastic ring [118] (Fig. 14.10). Despite the fact that some patients will have vaginal bleeding, prognosis for continuation of a pregnancy in which the vanishing twin phenomenon occurred, is excellent, regardless of the type of chorionic placentation. Birth weight, however, is lower for survivors of the vanishing twin syndrome [119]. One of the problems when this occurs is that serum aneuploidy screening may be affected with elevated levels of several analytes. In a recent study of 174 pregnancies with a vanishing twin, compared with control pregnancies, pregnancy associated plasma protein A (PAPP-A) increased by 21 % (p = 0.0026), alpha-fetoprotein (AFP) increased by 10 % (p < 0.0001), and dimeric inhibin A (DIA) increased by 13 % (p = 0.0470) in pregnancies with a vanishing twin. Unconjugated estriol and total human chorionic gonadotrophin were not significantly changed in these pregnancies [120]. Errors may also occur with noninvasive cell-free fetal DNA testing, specifically with sex determination [121]. Death of one fetus is somewhat similar to the vanishing twin phenomenon but generally occurring later in pregnancy [122]. Single fetal demise occurs in 3.7–6.8 % of all twin pregnancies and considerably increases the complication rate in the co-twin including fetal loss, premature delivery, and end-organ damage [123, 124]. In a large review of the literature, Ong and colleagues determined that following the death of one twin, the risk of a DC and MC co-twin demise was 4 and 12%, respectively. The risk of neurological abnormality in the surviving DC and MC co-twin was 1 and 18 %, respectively. The odds of MC co-twins intrauterine death was six times that of DC twins [122]. The issue of neurological damage in the surviving twin is particularly relevant to parents and clinicians. When death of one of a set of MC twins occurs, the surviving twin is at risk of major morbidity and mortality. This is thought to be due to exposure to thromboplastin, originating in the dead fetus circulation and reaching the surviving twin placental vascular connections and causing thromboembolic phenomena in various organs, particularly the brain [125] and DIC. Anomalies most commonly described in the literature all seem to involve some vascular accident component and include porencephalic cyst, hydranencephaly, microcephaly, intestinal atresia, gastroschisis, limb amputation, and aplasia cutis [126]. Another possible mechanism is hypovolemic-related hypotension, secondary to extensive blood loss from the surviving twin into the lower resistance circulation of the deceased twin. Fetus papyraceus is a rare condition with intrauterine demise of one twin [127]. The estimated frequency is 1:12,000 live births with an incidence of 1:184–1:200 twin pregnancies [128–130] but may occur more commonly in higher order gestations. Water content and amniotic fluid of the dead twin is reabsorbed and the fetus is compressed and mummified, resembling Egyptian parchment paper, hence the name. It is incorporated into the placenta of the surviving twin and is retained for various periods of time, including until delivery (preterm or term) of the surviving twin when it can be looked for in the placenta, after delivery [131].
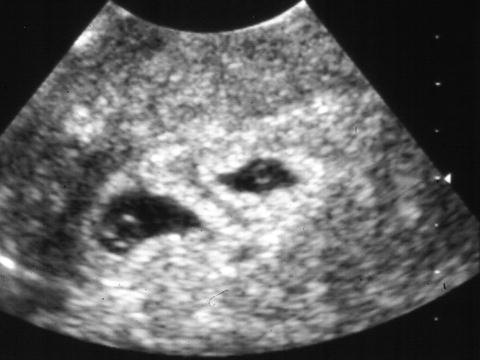

Fig. 14.10
Vanishing twin. One sac is much smaller and contains a very small yolk sac. If scanned at a later date, this would probably be missed
Growth Restriction and Differential Growth
In the first and second trimesters, the growth rate of normal twins is not significantly different from that of singletons. Any etiology of restricted growth in singletons may affect both twins equally or one, rather than the other, a condition designated as differential growth. Generally, in these cases, one twin is appropriate for GA (AGA) and one is small for GA (SGA). If the differential growth is secondary to one fetus being AGA and the other large for GA (LGA), this is not associated with major complications (at least until labor and delivery). The two major mechanisms for differential growth are placental specific dysfunction and genetic factors. In DZ twins, the SGA twin is often simply constitutionally small and different from his/her co-twin as two siblings might be. Another possible etiology is velamentous insertion of the cord of the small fetus since, as mentioned earlier, this entity is more common in multiple gestations [110] and is known to possibly be associated with intrauterine growth restriction [132], maybe due to disadvantageous competition for nutrients [133–136]. In addition, both fetuses may be SGA for placental or genetic reasons. In early pregnancy differential growth may be detected by a difference in crown-rump length (CRL). This trend may start very early [137]. A smaller than expected CRL is more commonly associated with chromosomal anomalies than a normal CRL [137–140]. Aneuploidy by chorionic villus sampling was 4.3 % in a group of singletons with smaller than expected CRL and 1.7 % in controls (p < 0.004) among 3194 chorionic villus sampling procedures, with 277 (8.7 %) fetuses with CRL smaller than expected by at least 7 days [138]. This association was demonstrated in a study of 159 twin pregnancies. Crown-rump length discordance of more than 10 % was associated with a significantly higher incidence of fetal anomalies (22.2 vs. 2.8 %; p = 0.01) [141]. Other outcomes, such as fetal loss are also worse with a 10 % discordance or more [142, 143], even in euploid fetuses [144]. In a large meta-analysis of 17 studies, twin pregnancies with CRL discordance ≥10 % were at significantly higher risk of perinatal loss (RR = 2.80, fetal loss at ≥24 weeks (RR = 4.07), BW discordance (RR = 2.24) and preterm delivery at <34 weeks (RR = 1.49) but not of fetal loss at <24 weeks [145]. Before 8 weeks, more than 3-mm difference is associated with 50 % risk of demise of smaller twin [146]. Such discordant growth is not always associated with poor outcome [65] and prediction of outcome based on this difference is less than optimal [147] but inter-twin CRL difference greater than 10 % increases the risk for discordant fetal growth or TTTS while CRL difference of less than 10 % carries an excellent prognosis in terms of perinatal outcome [148]. Growth discrepancy may also be found when both fetuses are AGA but one is significantly smaller than the other. The risk for adverse perinatal outcomes in these cases exists for monochorionic, but not dichorionic, twins [149]. Later in pregnancy (second and third trimesters), various definitions are used: estimated weight of one twin below the tenth percentile, abdominal circumference difference or growth discordance in estimated twin weights greater than 25 % [111, 150]. This aspect is beyond the scope of this book.
Discordance for Genetic/Structural Anomaly
See below, Screening for Genetic and Morphologic Abnormalities.
Complete Hydatidiform Mole and Coexisting Fetus
This is another rare “twinning” event with a normal fetus developing in the presence of a complete hydatidiform mole [151]. The incidence in 1:20,000–1:100,000 pregnancies [152] (Fig. 14.11). If the pregnancy is maintained, management is complicated and women should be followed in a high-risk obstetrics unit. Risks include fetal loss, preeclampsia and persistent gestational trophoblastic disease in over one-third of the cases [153, 154] but delivery of a healthy baby is not impossible, in approximately 50 % of cases [154, 155].


Fig. 14.11
Concomitant mole. Typical appearance of the placenta (black arrow). Fetal parts can be distinguished on the right (white arrow)
Complications Specific for MC Twins
There is, often, unequal sharing of the placenta, which may cause grave problems: discordant fetal growth with IUGR, metabolic compromise and death [156]. In addition, chronic unidirectional blood shunting through placental vascular anastomoses may occur and result in TTTS or twin reverse arterial perfusion [TRAP] and death). Furthermore, for MCMA twins, additional risks include conjoined twinning and cord entanglement. Risk of cerebral injury and subsequent cerebral palsy is seven times higher than in DC, most likely secondary to vascular anastomoses. If TTTS is present, this risk climbs to 21 % [56, 126]. After single intrauterine demise it is up to 18 % [157–159].
Twin-to-Twin Transfusion Syndrome (TTTS)
Twin-to-twin transfusion syndrome is one of the most serious complications of monochorionic multiple gestations that occurs in 10–15 % of MCDA twin pregnancies [19]. It is associated with a high risk of fetal/neonatal morbidity and mortality, close to 100 % if not diagnosed and managed [160]. Surviving fetuses are at risk of severe cardiac, neurologic, and developmental disorders. The diagnosis of TTTS requires two criteria: (1) the presence of a MCDA pregnancy; and (2) the presence of oligohydramnios (defined as a maximal vertical pocket of <2 cm) in one sac, and of polyhydramnios (a maximal vertical pocket of >8 cm) in the other sac [64]. Typically this syndrome is suspected when discordant fetal size is present, associated with polyhydramnios in the larger twin and oligohydramnios in the smaller twin of a MCDA pregnancy (Fig. 14.12). Changes in amniotic fluid volume are often the first sign, although CRL and nuchal translucency (NT) differences can also be seen, early in gestation [55, 161]. First trimester abnormal Doppler velocity in the ductus venosus (absent or reversed a-wave) has been associated with increased risks of chromosomal abnormalities, cardiac defects and fetal deaths [162] (Fig. 14.13). Differences in ductus venosus Doppler waveforms between twins has also been described as an early warning sign for subsequent development of TTTS [162, 163]. The etiology is unbalanced vascular anastomoses between the two placentae, arteriovenous (AV), arterioarterial (AA), venoarterial (VA), or venovenous (VV). While AA and VV anastomoses are on the surface of the placenta, AV and VA are deeper in the placental substance. Connections between the two circulations exist in virtually all MC placentation but TTTS develop in only 10–15 %, secondary to hemodynamic imbalance, a phenomenon that is not entirely explained [63]. In 150 pairs of MCDA twins, TTTS occurred predominantly in the presence of AV-anastomoses without compensating superficial AA-anastomoses (p = 0.005) and occurred more frequently in the presence of velamentous cord insertion [60]. There is relative hypovolemia in the smaller twin (donor) who releases vasopressin and renin–angiotensin, resulting in oligohydramnios. If this is extreme, the amniotic membrane becomes tightly adherent to the fetal body, resulting in an immobilized “stuck twin.” The other twin (recipient) becomes hypervolemic, which results in release of atrial natriuretic peptide (ANP) from the enlarged heart as well as brain natriuretic peptide (BNP). Release of these (natriuretic) hormones results in polyuria and polyhydramnios. In the recipient twin, hypervolemia and increased levels of renin and angiotensin (coming from the donor twin through transplacental crossing) result in cardiomegaly, hypertrophy, particularly of the right side and cardiomyopathy. Diastolic myocardial dysfunction occurs early in the pathophysiology of TTTS [164] and, together with cerebroplacental redistribution precede findings of overt cardiomyopathy [165]. Further deterioration occurs secondary to venous hypertension with development of hydrops. During the second trimester Quintero’s stages are often used to describe the severity of the condition [166].
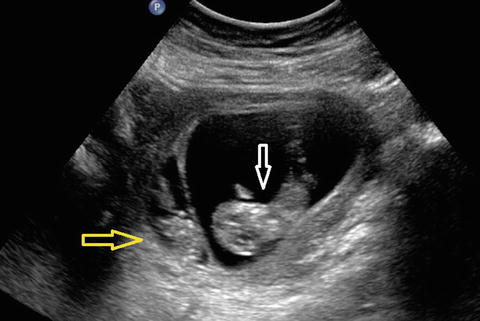


Fig. 14.12
Early signs of TTTS, 10 weeks. Clear difference in size and amount of amniotic fluid between donor (yellow arrow) and recipient (white arrow)

Fig. 14.13
Ductus venosus Doppler velocimetry in TTTS. Reversed a-wave is evident (arrow). This is a sign of cardiac failure in the recipient
Twin Anemia–Polycythemia Syndrome (TAPS)
TAPS is a form of TTTS, characterized by large inter-twin hemoglobin differences in the absence of amniotic fluid discordances, as opposed to twin oligo-polyhydramnios sequence or TOPS [167]. It may occur spontaneously in up to 5 % of monochorionic twins and may also develop after incomplete laser treatment in TTTS cases [168]. The etiology is probably few, minuscule AV placental anastomoses (diameter <1 mm) with a slow blood transfusion from donor to recipient, leading gradually to very high hemoglobin (Hb) levels in one twin and very low levels in the second one [167]. Diagnosis may be arrived at by finding discordance in fetal middle cerebral artery peak systolic velocity (MCA-PSV) measurements. Perinatal outcome is difficult to evaluate, since the literature contains mainly case reports and small series. Outcome vary according to severity and may range from double intrauterine fetal demise to two healthy neonates without major morbidity at birth, besides large inter-twin Hb differences. Severe anemia can be seen at birth in the donor, requiring blood transfusion, and severe polycythemia in the recipient, requiring partial exchange transfusion. Cases of severe cerebral injury in TAPS have also been described but outcome seems to be much better than in classic TTTS. In 19 pairs of twins affected by TAPS, matched to 38 pairs of non-affected twins, neonatal mortality and morbidity rates were similar to controls [168].
Twin Reversed Arterial Perfusion (TRAP) Syndrome
This is a very severe form of TTTS complication occurring with monochorionic placentation, due to unidirectional arterio-arterial placental anastomosis. It can be diagnosed in the first trimester [169]. It affects about 1 % of MC twins, with a prevalence is 1:35,000 births. There are two theories to explain the phenomenon, both resulting in artery-to-artery anastomosis between the umbilical arteries of both twins. One theory states that the primary event is a teratological accident with severe abnormal development of the fetal heart, resulting in absence of the structure (hence “acardiac”). The vascular anastomoses are felt to be secondary. According to the second explanation, the primary event is the development of anastomoses and reversed blood perfusion from the donor (pump) fetus to the acardiac (recipient) twin, as demonstrable by Doppler studies [170, 171] (Fig. 14.14). This is responsible for secondary fetal cardiac hypoplasia [172] and amorphic development of one twin with poor formation of the head, trunk, and upper extremities but, occasionally recognizable spine and lower extremities. The reason for this differential development is that deoxygenated blood from the pump twin crosses the placenta through artery-artery anastomosis, resulting in retrograde flow to the acardiac fetus. The lower part of the body extracts the remainder of the oxygen, allowing for some development of the lower limbs, while the remainder of the body gets none. Acardiac twins often demonstrate a two-vessel cord and polyhydramnios. A somewhat older classification includes acardius amorphous, the least differentiated, appearing as a heterogenous mass, acardiac acephalus, the most common form of acardia, where the fetus lacks a head, thorax, and upper extremities (see Fig. 14.14), as well as acardius acornus and acardius anceps, the most developed form, with a head, thorax and abdominal organs but no heart. All acardiac twins may originate in the acardius anceps which evolves into the others because of poor oxygen supply to the remainder of the fetus. TRAP occurs in both MCMA and MCDA twin pregnancies. The overall pregnancy loss rate is estimated at 50 %, due to high output cardiac failure in the pump twin and preterm delivery [71]. Prognosis can be ascertained by calculating the ratio of the acardiac weight to pump twin estimated weight. The weight of the acardiac twin is calculated by the formula: weight (g) = 1.2 × (longest dimension (cm))2 − 1.7 × longest dimension (cm) [173]. If the ratio is above 70 %, this indicates dire prognosis, as do signs of congestive heart failure (such as non-immune hydrops) in the pump twin. Various treatment modalities have been described: cord occlusion (by embolization, cord ligation, laser coagulation, bipolar diathermy, and monopolar diathermy and intrafetal ablation (by alcohol, monopolar diathermy, interstitial laser, and radiofrequency) with intrafetal ablation appearing to provide the best results [174].


Fig. 14.14
TRAP sequence. (a) The acardiac twin is in the upper part of the image, as marked. Some vague anatomy can be recognized. (b) Doppler velocimetry demonstrates flow away from the transducer in the umbilical artery of the acardiac twin, i.e., reversed, from the placenta towards the fetal body
Conjoined Twins
Twinning occurs in approximately one of every 87 live births. One-third of these are monozygotic twins and about 1 % of monozygotic twins are conjoined. Conjoined twins represent a rare entity with estimates ranging from 1 in 75,000 to 1 in 250,000 deliveries [22, 23]. In the USA, the incidence is 1 per 33,000–165,000 births and 1 per 200,000 live births [175]. Conjoined twins are MCMA with the diagnosis usually made in the second trimester, although early, first trimester diagnosis is also feasible [176, 177]. They are more common among females than males (3:1 in live born), and in nonwhites than whites [178]. For unclear reasons, it seems to be more common in Indian and African population. Stillbirth rate is very high (40–60 %). More cases are being reported now because of the routine use of ultrasound in early pregnancy [179–181]. Conjoined twins may be symmetrical with two well-developed bodies or asymmetrical where one is normally developed and the second is incomplete, for example twin reversed arterial perfusion or TRAP (previously called acardiac twin) or parasitic twin or fetus in fetu, a very rare condition where a monozygotic, MCDA abnormal twin with rudimentary anatomy is contained within a host twin [182].
Classification of conjoined twins is according to the site of union [183]. The most common types are the following:
1.
Thoracoomphalopagus (joined at chest or abdomen or both), 75 % (Fig. 14.15). Thoracopagus generally share a heart (Fig. 14.16), which renders separation to save both twins virtually impossible.



Fig. 14.15
Conjoined twins, 13 weeks. This is a typical thoraco-omphalopagus, the most common type, with joining at the thorax and abdomen levels

Fig. 14.16
Conjoined twins, 8 weeks. Color Doppler confirms conjoined twins with one heart
2.
Pyopagus (joined at the buttocks), 18 %.
3.
Ischiopagus (joined at the ischium), 6 %.
4.
Craniopagus (linked at the cranium), 2–5 %.
Cord Entanglement
This complication of MZ twins (designed as uniovular) was already described (not by ultrasound!) in 1952 [187]. It may begin early in the pregnancy, as soon as fetal (nonvoluntary) movements are initiated, around 7–8 weeks GA [188]. Major risks include intermittent cord compression which may result in neurological damage although a direct cause-effect relation is hard to prove [73] and complete occlusion with fetal demise [189]. Ultrasound is very useful to detect this condition specifically with the use of spectral and color Doppler. Color Doppler demonstrates a complex vascular mass [190, 191] (Fig. 14.17). Three-dimensional ultrasound can also be used to demonstrate the entanglement [192, 193]. There are several Doppler waveform characteristic of entanglement: persistent absent end-diastolic velocity in the umbilical artery [194] and pulsatile, high velocity waveform, with absent diastolic in the umbilical vein [195]. A notch in the umbilical artery before the entanglement region, indicating downstream elevated resistance was described as a specific sign associated with bad prognostic [196, 197], although more recent studies seem to indicate that the presence of an umbilical artery notch in cases of cord entanglement, without other signs of fetal deterioration, are not indicative of an adverse perinatal outcome [75].
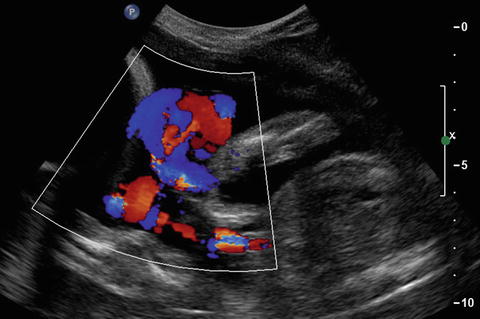

Fig. 14.17
Cord entanglement in monoamniotic twins. In this color Doppler image, a mass containing both cord is appreciated. The gestation is not in the first trimester but in the early second trimester
The previously cited dire prognosis [189] may, in fact, be less dire than originally described [198]. In a study of 114 monoamniotic twin sets (228 fetuses) with documented cord entanglement at delivery, cord entanglement itself did not contribute to prenatal morbidity and mortality [199]. In another report, umbilical cord entanglement was present in all 18 sets of monoamniotic twins when it was systematically evaluated by ultrasound and color Doppler [73]. Perinatal mortality was mainly a consequence of conjoined twins, TRAP, discordant anomaly, and spontaneous miscarriage before 20 weeks’ gestation.
Screening for Genetic and Morphologic Abnormalities1
Twins are at increased risk for genetic anomalies, as clearly documented in a study of 5.4 million births, from 14 European countries, of which 3 % were multiple [200]. The risk of karyotypic anomalies is different between monozygotic and dizygotic twins. For MZ twins, the age-related risk to simultaneously be abnormal is the same as in a singleton gestation, although, from a maternal standpoint, the risk to the pregnancy to have one affected fetus is twice the risk of a singleton in cases of twins, three times in the case of triplets, etc. For dizygotic twins, however, the risk of one being affected is similar to a singleton but the risk of both being affected is much lower. In fact it is the square of the risk of a singleton: for instance if the age related risk of the mother is 1:250, the risk of both twins, if dizygotic, to be affected is 1:250 × 1:250 or 1:62,500 [201]. Screening for trisomy 21 in multiple gestations is complicated [202, 203]. Local prevalence of aneuploidy in twins needs to be taken into account for all calculations of risk [204]. Serum screening alone is of limited value because a high value may indicate elevated risk but with no determination of which or how many fetuses are affected, since it is possible that an unaffected co-twin may “mask” the abnormal serum results of an affected one and the fact that for DZ or MZ twins the interpretation may need to be different [205–207]. Specific references may need to be utilized [208]. An acceptable screening test for aneuploidy in the first trimester twin pregnancy includes fetal nuchal translucency, combined with maternal age. Structural (as opposed to maternal serum) first trimester markers (including NT, nasal bone, tricuspid valve flow, and ductus venosus waveform) may be helpful in risk assessment for aneuploidy as they are independent measurements for each fetus, regardless of chorionicity [209]. Nuchal translucency (NT) screening is effective and is an excellent modality (when cell free fetal DNA is not available, see below) for twin pregnancies. When screening is done by nuchal translucency and maternal age, a pregnancy-specific risk should be calculated in MC twins. In DC twins, a fetus-specific risk is calculated [203]. Among twins, NT alone has a 69 % trisomy 21 detection rate [210]. Screening with first trimester serum analytes, combined with nuchal translucency (also known as First Screen) may also be considered. It decreases the false-positive rate. In a 2014 systematic review of first trimester combined risk assessment (nuchal translucency and maternal serum analytes) in twin pregnancies, test sensitivity in DC and MC twins was 86 % and 87 %, respectively [211]. Integrated screening with First Screen and second trimester serum screening is an option. Naturally, in addition to trisomy 21, increased nuchal translucency is a marker for other aneuploidies, congenital malformations, and a sign of early development of TTTS [65, 161]. First-trimester combined NT and serum biochemistry has a 72 % DS detection rate, and an integrated screen will have an 80 % DS detection rate at a 5 % FPR [210]. The issue of “vanishing twin” (see below) is specifically problematic since early loss of one or more embryos of a multiple gestation may affect analyte levels [212]. In known cases, NT screening may be the preferred option. When screening is done by nuchal translucency and maternal age, a pregnancy-specific risk should be calculated in MC twins. In DC twins, a fetus-specific risk is calculated [203]. Some laboratories that perform noninvasive DNA screening (NIDS) with MPS methodology offer testing for twin gestations after it has been validated for twins. Testing for monozygotic twins is expected to perform similarly to a singleton gestation, although testing in dizygotic twin and higher-order multiple gestations is complicated by the fact that the per-fetus fetal fraction may be lower [213]. In fact, the non-reportable rate is higher (7.4 %) than that for singleton pregnancies (2 %). Additionally, if one fetus is euploid while the other is aneuploid, there is a dilution of the cell free fetal DNA from the aneuploidy fetus resulting in decreased detection rates compared to singleton gestations. Based solely on NIDS results, it is impossible to determine which twin is abnormal. Therefore, invasive testing (CVS or amniocentesis) is required to distinguish which twin is affected. Several false-positive results have been reported with biological basis, such as confined placental mosaicism (CPM), maternal chromosome abnormality and vanishing twin. Additional unexpected information such as undiagnosed molar pregnancy or vanishing twin may be detected by some NIDS methods [214].
The incidence of congenital anomalies is much higher in MZ twins, in fact three to five times higher than in DZ twins [215, 216]. Although this has been party correlated with assisted reproductive technologies [217], there seems to also be a direct relation with the twinning phenomenon itself, whether spontaneous or induced with a common etiology for both the MZ twinning and the early sequence of the malformation [218]. Most common structural anomalies in twins include anencephaly, facial clefts, holoprosencephaly, VATER association (vertebral defects, imperforate anus, esophageal fistula with tracheoesophageal fistula, radial and renal dysplasia), exstrophy of the cloaca malformation sequence, and sacrococcygeal teratoma, all of which should be recognized early by detailed ultrasound anatomy scan. In a large study by Glinianaia and colleagues 2329 twin pregnancies (4658 twins) and 147,655 singletons were compared. The rate of congenital anomalies in twins was 405.8 per 10,000 twins versus 238.2 per 10,000 singletons (rate ratios [RR] = 1.7, 95 % confidence interval [CI] 1.5-2.0]. In twins with known chorionicity (84.8 % of all twins), the prevalence of congenital anomalies in MC twins (633.6 per 10,000) was nearly twice that in DC (343.7 per 10,000; RR = 1.8, 95 % CI 1.3-2.5). There was an increased rate of congenital anomalies for all major types of anomalies in twin compared with singleton pregnancies, except chromosomal abnormalities [215]. Monozygocity specifically MCDA twinning, seems to be an independent factor for an increase in congenital heart disease (CHD) with a 9.18 relative risk increase in one report of 40 fetuses with CHDs among 830 fetuses from MCDA twin gestations [219]. Congenital heart disease, however, is also more common in DZ twins than in singleton [220]. Thus fetal echocardiography is, in fact, indicated in all wins. In a study of 844 pairs of twins, the prevalence of major congenital malformations was 2.7 % for MZ twins, 1.0 % for DZ twins, and 0.6 % for singletons. The concordance rate of major congenital malformations was 18 % for MZ twins, but no DZ pair was concordant for any major congenital malformation [221].
Are monozygotic twins “really” identical? They are very similar but genetically, most often, not “exactly” the same [222–224]. In fact, hundreds (360 by one estimate) of genetic differences may occur very early in fetal life. These may be due to post-fertilization events, such as chromosomal mosaicism, skewed X-inactivation, imprinting mechanisms, as well as DNA point mutations or copy errors, taking place early after blastocyst splitting [224]. There are also, genetic differences due to mutations which may occur later in life as well as epigenetic2 modifications, due to environmental factors [225, 226]. Another phenomenon explaining a difference in the karyotype of two MCMA twins is heterokaryotypia: a discordance in karyotype due to either an early postzygotic chromosomal rescue in one fetus or a mitotic error that leads to one trisomic fetus with a normal co-twin [227]. Discordance for a congenital anomaly is extremely problematic, from a moral, ethic, religious, philosophical, and, often, medical standpoints [228, 229]. Until intrauterine therapy is effective and safe (it may already be for a very small number of anomalies), the options include expectant management [230], termination of the entire pregnancy or selective feticide [231–233]. Selective termination of an anomalous DC twin is relatively safe with intravascular injection of potassium chloride or digoxin, although there is some increased risk of miscarriage or preterm delivery [234, 235]. In monochorionic twins, selective feticide needs to result in complete separation of the circulations [236, 237] and is, thus, best accomplished by sealing one umbilical cord with ligation [238], bipolar coagulation [239, 240], radiofrequency [241], or laser ablation [236].
Maternal Complications
As described in the introduction, maternal morbidity (and mortality) is increased in multiple pregnancies. Multiple pregnancy is the most powerful predictive factor for adverse maternal, obstetrical, and perinatal outcomes [242]. Pregnancy induces physiological stress to the maternal body and multiple gestations provide even additional strain and nutritional demands [243]. Most of the complications do not become clinically apparent in the first trimester but later in pregnancy, such as preeclampsia and diabetes [244]. Among women with 684 twin and 2946 singleton gestations enrolled in multicenter trials, rates for both gestational hypertension and preeclampsia were significantly higher among women with twin gestations than among those with singleton gestations. Furthermore, adverse neonatal outcomes were more frequent in women with twin pregnancies and hypertensive complications [16]. In a study of over 23,000 women, 553 of whom had twins, after adjusting for age, race/ethnicity, body mass index, maximal systolic and diastolic blood pressure, smoking and parity, multiple regression analysis showed that twin pregnancy was associated with an approximately twofold increase in the risk for developing gestational diabetes. The risk was highest among African-American and young women [245]. Thromboembolic disorders are major causes of morbidity and mortality in the pregnant patient. Contributing factors are increased blood coagulability [246], elevated BMI, maternal age above 35 and, specifically, multiple gestation with an incidence rate of 6.3/10,000 year in singletons versus 18.2/10,000 year among women with multiple pregnancies [247]. Other complications more common in women carrying multiple gestations, most likely secondary to increased levels of various hormones, in particular βHCG, include hyperemesis gravidarum [248]—although this is not universally accepted as a more frequent complication in multiple pregnancies [249]—iron deficiency anemia [250], intrahepatic cholestasis of pregnancy [251] and pruritic urticarial papules and plaques of pregnancy or PUPPP. This is the most common specific dermatosis of pregnancy, with an incidence is 1/160–1/300 pregnancies [252]. The majority of patients are nulliparous and PUPPP is 8- to 12-fold more common in women with multiple gestations, possibly due to increased hormones levels, as stated above, or increased abdominal distension [253]. An additional complication is acute fatty liver. This is a rare condition, usually of the third trimester, complicating approximately 1 in 10,000 singleton gestations [254] but, of all the published cases, 14 % have been reported in twin gestations [255]. The rate seems to be 7 % in triplet pregnancies [256].
Higher Order Multiple Gestations
These pregnancies (triplets, quadruplets, etc.) are at extremely high risk of complications [257]. The classic teachings are that the prevalence for triplets is 1:902 and 1:903 for quadruplets. Numbers have greatly changed with the introduction of ART [258–260]. Classification is based on chorionicity and amnionicity [261] (Figs. 14.18 and 14.19). In a study of 49 consecutive sets of triplets, including 18 sets of spontaneously conceived triplet pregnancies and 31 sets resulting from ART the rate of MZ twin pairs was 48 % among spontaneously conceived triplet pregnancies; 30 % of DC triplet pregnancies were MZ and 70 % DZ; 20 % of trichorionic (TC) triplet pregnancies were DZ and 80 % trizygotic (TZ). For triplet pregnancies conceived using ART, the rate of MZ twin pairs was 6.5 %; 100 % of DC triplet pregnancies were DZ; 4 % of TC triplet pregnancies were DZ and 96 % TZ [261]. Early complications, such as genetic anomalies, growth discordancy, TTTS are similar to twin pregnancies, depending on placentation, although, naturally, much more challenging from a management standpoint [258, 262, 263]. Incidence of congenital anomalies is not increased, compared to twins [259]. The complications are mostly later in pregnancy. In a study of 316,696 twin, 12,193 triplet, and 778 quadruplet pregnancies, compared with mothers of twins, mothers of triplets and quadruplets were more likely to be diagnosed with preterm premature rupture of membranes (AORs, 1.53, 1.74, respectively), pregnancy-associated hypertension (AORs, 1.22, 1.27), and excessive bleeding (AORs, 1.50, 2.22), to be delivered by cesarean section (AORs, 6.55, 7.38) at <29 weeks of gestation (AORs, 3.76, 7.96), and to have one or more infants die (AORs, 3.02, 4.07). The rate of maternal complications is also increased compared to twin pregnancies where it is already increased compared to singletons. In a retrospective study of 57 triplet gestations, preterm labor occurred in 86.0 %, anemia in 58.1 %, preeclampsia in 33.3 %, preterm premature rupture of the membranes in 17.5 %, postpartum hemorrhage in 12.3 %, and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome in 10.5 % [264].