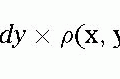Myocardial Perfusion and Viability
Robert W. W. Biederman
MYOCARDIAL PERFUSION
Primarily, this chapter will focus on using cardiovascular magnetic resonance (CMR) to understand myocardial viability; that is the detection and distinction between scar and recoverable myocardium. We note that, myocardial perfusion strategies have predated the more recent use of delayed hyperenhancement (DHE). A considerable amount of data has been amassed concerning perfusion sequences designed to detect single, double, and triple vessel coronary artery disease (CAD) in a manner not dissimilar to current nuclear strategies. However, CMR differs strikingly from nuclear metabolic radionuclide tracer methods, because CMR perfusion strategies detects alterations in myocardial blood flow (typically related to epicardial vessels) whereas nuclear methods are sensitive to radiotracer interaction with a particular cellular domain, related to integrity of either cellular membranes or metabolism. Distinct differences exist chiefly in that CMR requires no radionuclide injection, has higher imaging resolution than current nuclear techniques, and retains the ability to detect subendocardial defects, and appears able to detect microvascular disease, especially in women, in the absence of epicardial disease.
CMR contrast agents, which are typically gadolinium based-chelates, generally have similar relaxivity rates, with the possible exception of for gadobenate dimeglumine (Gd-BOPTA), which produces approximately twice the 1/T1 effect. The critical action of contrast agents is that they increase the resident myocardial signal because of changing the T1-relaxation rate, and are generally safe in their pharmacokinetics and metabolism when cleared from the body by normally functioning kidneys.
Multiple methods for analyzing and processing CMR perfusion data exist. Typically, the first pass contrast kinetics are visually detectable, allowing comparison of rest and stress perfusion images. Alternatively, strategies exist to quantify myocardial uptake, using prototypic algorithms, most incorporating a measure of the input flow waveform to the myocardium, allowing normalization for underlying cardiac function. Typically, myocardial segmental analysis is performed on the slope of the time intensity curve. Myocardial segment time intensity curve metrics are compared for differences to determine the likelihood for territorial disease within the myocardium. More recently, attempts to improve the already reasonable sensitivity and specificity has led to considerations of double bolus strategies utilizing a low, followed by a high, dose contrast administration.
Paradoxically, low-dose gadolinium for detecting perfusion defects yields the best receiver-operator-characteristic (ROC) curve when using angiography as the “gold standard” for the detection of CAD. Therefore, doses of 0.5 mmol/kg are used to (a) maximize accuracy; (b) increase reproducibility among readers; (c) reduce susceptibility artifacts, which are a major source of error, especially at the interface between low and high regions of concentration of gadolinium such as those that exist along the intraventricular septum; (d) detect perfusion defects that exist at least 8 seconds after contrast injection in two or more contiguous slices, which has been shown to be a measure of maximum sensitivity in recent studies; and (e) detect microvascular disease.
CMR stress testing has now become an accepted protocol, with the following being the indications for stress testing:
Any suspicion for CAD
Inability for standard stress testing requiring exercise (treadmill)
Poor quality of other invasive or noninvasive study
Preoperative risk assessment
Contraindications for stress testing include the following:
Allergy to dobutamine or adenosine (or provoking agent)
Unstable angina
Uncontrolled arrhythmias
Severe aortic stenosis
Aortic dissection
Large (>55 mm) thoracic or (>45 mm) abdominal aortic aneurysm
Acute or subacute myocarditis
Acute or subacute pericarditis
Severe hypertension (>225 mm Hg or >120 mm Hg, systole or diastole, respectively)
Reactive airway disease (adenosine)
Bradycardia and/or advance arteriovenous (AV) block (>second degree Mobitz type II)
Myocardial infarction (MI) within last 3 days
Numerous protocols are available for stress testing:
Adenosine: IV infusion at 0.14 mg/kg/minute for 4 minutes and 1 to 2 minutes extra during imaging
Dipyridamole: Infusion of 0.56 mg/kg for 1 minute followed by a 4 minutes delay while endogenous levels of adenosine build up in the body
Dobutamine: Infusion at variable dose escalating strategies, generally 5, 1, 20, 30, and 40 µg/kg/minute for 3 minutes at each dose (atropine 0.25 mg to achieve age-predicted maximal heart rate (may repeat up to 4 times)
It is important to monitor the patient during stress testing to detect any untoward event:
Heart rate, blood pressure (BP), pulse oximetry, and electrocardiogram (ECG) (note: due to the magnetohydrodynamic effect marked blood flow-related changes render T-wave monitoring ineffective)
Constant contact with patient through visual video monitoring, cameras, and hand alarm (for patient to squeeze and alert imaging staff)
CMR monitoring for wall motion abnormalities
Keeping stocked crash cart nearby, and trained personnel ready to deal with the inevitable emergency that presents on stressing this intermediate- to high-risk group (ideally, a nurse is on standby during these cases, especially if risk is elevated) Typically, the code team are several minutes away from the CMR center, necessitating a high competency level with cardiopulmonary resuscitation (CPR) (advanced life support [ALS]). The physician in charge must have advanced and current basic cardiac life support (BCLS) training.
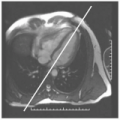
DHE imaging is often performed following perfusion imaging for viability evaluation. Gadolinium (see Fig. 12-1) is introduced that accumulates in infarcted tissue. The contrast agent is a chelate that decreases T1 of blood approximately threefold when administered IV with the following ideal characteristics:
Inert
Safe, when cleared from the body by normal kidney function
Remains predominantly interstitial
High degree of discrimination between normal and infarct tissue
High degree of sensitivity and specificity (>98%) in detecting MI
Can image in less than 30 minutes
Can be combined with LV function analysis
Acute or chronic imaging
High reproducibility
Since 2003, the reference standard for viability
CASE STUDIES
Case Study 1.
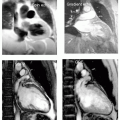
DHE pre-percutaneous transluminal coronary angiography (PTCA) in a 56 year-old white man presenting with an acute anterior wall MI (see Fig. 12-1). The impact of a large type IV proximal left anterior descending artery (LAD) infarct is demonstrated with a large territory of nearly circumferential myocardial involvement.
Case Study 2.
DHE in the same patient as in case study 1 at 6 months following PTCA, demonstrating marked improvement in the area of scar (see Fig. 12-2). The area at risk is substantially less in the very acute setting than in the 2 to 3 days post-MI. The impact of the acute revascularization intervention is demonstrated by comparison of the DHE regions pre- and post-MI.
Case Study 3.
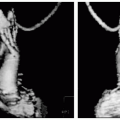
The integration of structure and function is easily performed by combining the steady state free precession (SSFP) and DHE images, taking less than 25 minutes to perform for both series in a 49-year-old woman with nausea and vomiting as her first presentation of this right coronary artery infarction (see Fig. 12-3). In the bottom right panel, note the mostly subendocardial infarction pattern of the basal and mid inferior wall that becomes more transmural at the inferior apex (chevron). This strongly supports a large amount of viable myocardium. The subendocardial extent (<25%) is best visualized in the lower right panel (arrow).
Case Study 4.
A 39-year-old white man presents 1 year after a large MI, seeking a second opinion regarding consideration for undergoing a sixth PTCA for continued chest pain. A search for viability was performed (see Fig. 12-4). Perfusion imaging shown in lower left panel reveals a dense hypointense region, nearly perfectly superimposed on the region of DHE scar, suggesting little ischemia. The extent of scar was nearly transmural except for a small region in the mid-anterior and inferior septum, for which he had recently under gone several smaller vessel PTCA (posterior descending artery [PDA] and right ventricle [RV] marginal branches) with minimal resolution of his symptoms. No further interventions were recommended and the patient was placed on ranolazine, 500 mg (CVT) with complete relief of his symptoms in 6 weeks. He is chest pain free 2 years later.
Case Study 5.
The ability to resolve subendocardial defects are substantially better with CMR than with nuclear imaging owing to the improvement in spatial resolution. As shown in this Fig. 12-5, this 41-year-old man with a 1-mm ST elevation MI 1 week previously, and negative nuclear scan performed 1 day post-MI, has clear evidence for a lateral wall MI. This demonstration underscores the utility of CMR evaluation by DHE imaging to determine chest pain etiology in the absence of cardiac catheterization in certain populations.
Case Study 6.
Direction of intervention services is depicted in this 64-year-old white woman with chest pain and two-vessel CAD, both with 80% proximal lesions. By perfusion imaging (see Fig. 12-6, lower right panel) only the PDA territory demonstrates hypoperfusion at rest, directing interventional efforts to that vessel, sparing the left circumflex artery, whereas both had by angiography, suitable indication. Note the extension of ischemia well beyond the scar, supporting the notion of intervention. The DHE imaging demonstrates that there will be a return of function given that <50% scar is present.
Case Study 7.
A 65-year-old white woman with acute inferior wall MI, left ventricular ejection fraction (LVEF) of 25% by catheterization, and high-grade three-vessel disease, referred for viability assessment before performing a moderately high-risk coronary artery bypass graft (CABG) (see Fig. 12-7). The perfusion defect is in agreement with catheterization, in that there is high-grade resting hypoperfusion (worse in the inferior wall) indicating extensive CAD. The DHE images demonstrate a near transmural defect confined to the territory of the right coronary artery lesion, with otherwise viable myocardium. This is a very favorable pattern to support an excellent return of segmental function to a large amount of myocardium and substantial improvement of her postoperative ejection fraction (EF) at 6 weeks (EF 44%). Note the subtle RV sub endocardial infarct pattern curve.
Case Study 8.
A 73-year-old black man with equivocal sestamibi perfusion read finally as negative, referred for evaluation by CMR




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree