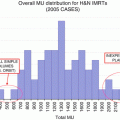
Fig. 8.1
Dosimetric comparison of treatment plans for intensity-modulated radiation therapy (IMRT) vs. 3D-conformal RT vs. traditional RT. Axial dose distributions through the center of the nasopharynx and neck for IMRT (left), 3D-conformal RT (middle), and traditional treatment plans (right). Note the relatively poor coverage of the skull base and medial nodal regions in the traditional plan and the improved dose conformality of the IMRT plan (From: Hunt et al. [4], with permission from Elsevier)
8.2.2 Salivary Function and Treatment Compliance
The most common complication of RT for NPC is a decline in salivary function, known as xerostomia, due to a damage of nearby salivary structures. The symptoms of xerostomia can significantly affect a patient’s quality of life [6]. The severity of xerostomia depends mostly on the dose and volume of salivary gland within the radiation field. Dosimetric comparisons have revealed that a mean dose of 26 Gy or less to the parotid glands is necessary to preserve salivary function [7, 8].
The main benefit of IMRT over conventional RT for NPC is the ability to spare the parotid glands. Two phase III randomized controlled trials assigned patients to receive either 2D RT or IMRT with parotid-sparing techniques and evaluated outcomes at 1 year after treatment. The first trial found that IMRT was associated with superior quality of life outcomes [9]. The second study also found benefits in observer-rated xerostomia outcomes and preservation of parotid function (measured by parotid flow rate) with the use of IMRT [10], as well as a trend towards improvement in patient-reported xerostomia outcomes. The same study revealed the somewhat surprising finding that xerostomia quality of life scores only correlated weakly with both salivary flow rates and observer-rated xerostomia outcomes. Therefore, evaluation of both patient-reported and physician-reported outcomes remains important. Regardless, both phase III studies showed improved xerostomia outcomes with the use of IMRT compared with conventional 2D RT.
The lesser toxicity associated with IMRT may also improve treatment compliance or the ability of patients to tolerate the prescribed therapy. A multi-institutional trial of IMRT by the Radiation Therapy Oncology Group, RTOG 0225, showed that 90 % of patients were able to receive the full 70-Gy prescribed dose and that 88 % of the patients with T2b or higher or N+ disease were able to receive the full three cycles of concurrent cisplatin [11]. These findings compare favorably to previous studies that used conventional RT techniques, for example, chemotherapy compliance rates were 63 % in the Intergroup 0099 trial, 71 % in a Singapore randomized trial, and 52 % in the Hong Kong NPC-9901 trial [3, 12, 13].
8.2.3 Disease Control
In addition to improving toxicity outcomes, excellent disease control outcomes have been reported by several institutions. Lee et al. reported findings from an initial series of patients with NPC treated with IMRT, with an incredible 4-year local control rate of 97 %, despite 70 % of patients in that study having locally advanced disease [14]. Kwong et al. reported the first prospective series, with 3-year outcomes of 100 % local control (LC), 92.3 % regional control, and 100 % overall survival (OS) rates [15]. These excellent outcomes are supported by additional published series from many individual institutions, comprehensively reviewed by Wong et al. (Table 8.1) [16]. The RTOG 0225 trial further demonstrated the feasibility of implementing IMRT techniques across the multiple US institutions [11]. That phase II study reported excellent 2-year outcomes of 93 % LC, 89 % local-regional control (LRC), and 80 % OS rates (Table 8.1).
Table 8.1
Published studies of IMRT for nasopharyngeal cancer
Reference | Stage | No. of patients | Median follow-up time, mo | Time point, y | Local control rate, % | Regional control rate, % | Freedom from DM rate, % | Overall survival rate, % |
|---|---|---|---|---|---|---|---|---|
Lee et al. (2002) (UCSF) [14] | All | 67 | 31 | 4 | 97 | 98 | 66 | 88 |
Kwong et al. (2004) (Hong Kong) [15] | T1 N0–1 | 33 | 24 | 3 | 100 | 92 | 100 | 100 |
Kam et al. (2004) (Hong Kong) [22] | All | 63 | 29 | 3 | 92 | 98 | 79 | 90 |
Wolden et al. (2006) (MSKCC) [24] | All | 74 | 35 | 3 | 91 | 93 | 78 | 83 |
Kwong et al. (2006) (Hong Kong) [23] | III–IVB | 50 | 25 | 2 | 96 | NA | 94 | 92 |
Lee et al. (2009) (RTOG 0225) [11] | All | 68 | 31 | 2 | 93 | 91 | 85 | 80 |
Lin et al. (2010) (China) [25] | IIB–IVB | 370 | 31 | 3 | 95 | 97 | 86 | 89 |
Lee et al. (2012) (RTOG 0615) [19] | IIB–IVB | 42 | 30 | 2 | NA | NA | 91 | 91 |
Notably, other factors may contribute to improvements in LRC associated with IMRT, including the use of chemotherapy, better supportive care, and technologic advances in imaging that provide better tumor delineation. Other limitations associated with historical comparisons include changes in the criteria for disease staging over time as well as improved staging with the use of magnetic resonance imaging (MRI) and positron emission tomography (PET) [17]. For example, because MRI is more sensitive than computed tomography (CT) for detecting minimal skull base involvement or intracranial extension, the T category tends to be upstaged when MRI is used rather than CT.
8.3 Techniques
8.3.1 Diagnostic Work-Up for Target Volume Delineation
Disease staging should include both CT and MRI of the head and neck. CT is important for assessing cortical bone involvement; MRI provides superior visualization of skull base involvement and tumor invasion into soft tissue structures compared with CT [18]. Infiltration of disease into the bone marrow is best seen as hypodense regions relative to normal marrow on T1-weighted non-contrast MRI scans. Fusion of the skull base portion of the CT scan with the MRI scan should aid in delineating the gross tumor volume (GTV). MRI also allows retropharyngeal lymph nodes to be distinguished from primary tumor, whereas CT may not.
Enlarged retropharyngeal lymph nodes should be considered a gross disease. Involvement of other lymph node regions is suggested by the presence of central necrosis, extracapsular spread, or nodal diameters of 1 cm or more. PET/CT may help to clarify involvement of borderline lymph nodes. Generally, because NPC has a high likelihood of nodal spread, any nodes suspected of harboring disease should be considered a gross disease.
8.3.2 Simulation and Daily Localization
The patient should be set up for treatment simulation supine, with the neck extended. The immobilization device to be used should include at least the head and neck; if possible, shoulders should also be immobilized to ensure the reproducibility of patient setup from day to day, especially when an extended-field IMRT plan is to be used. A bite block can be placed during treatment simulation and throughout treatment to move the tongue away from the high-dose regions in the nasopharynx.
CT-based treatment simulation should involve 3-mm-thick scan slices with intravenous contrast to help delineate the GTV, particularly the lymph nodes. The isocenter is typically placed immediately above the arytenoids. Image registration and fusion applications with MRI and PET should be used to help delineate target volumes, especially regions of interest that encompass the GTV, skull base, brainstem, and optic chiasm.
8.3.3 Target Volume Delineation and Treatment Planning
Several IMRT dose-fractionation regimens have been used for NPC (Table 8.2). Excellent LRC rates in excess of 90 % have been reported with the use of these regimens.
Table 8.2
IMRT dose and fractionation schemes
Dose and fractionation | Study institution and reference | |||
|---|---|---|---|---|
RTOG [19] | Fujan [20] | SKL [43] | PWH [22] | |
Gross disease, Gy | 69.96 | 66.0–69.75 | 68 | 6,674 |
Gross disease, Gy/fraction | 2.12 | 2.2–2.25 | 2.27 | 2 |
High-risk region, Gy | 59.4 | 60–60.45 | 60 | 60 |
High-risk region, Gy/fraction | 1.8 | 1.95–2.0 | 2 | 1.82 |
Low-risk region, Gy | 50–54.12 | 54–55.8 | 50–54 | 54–60 |
Low-risk region, Gy/fraction | 1.64–2.0 | 1.8 | 1.8–2.0 | 2 |
Margin around GTV, mma | 10 | 8–13 | NA | 13 |
Several acceptable definitions of target volumes, including the GTV, the clinical target volume (CTV), and planning target volume (PTV), have been used at different institutions, as reviewed by Wong et al. [16]. The RTOG established a guideline for target volume delineation with RTOG 0225, which was successfully implemented in that multi-institutional study [11]. Suggested target volumes for the GTV and high-risk CTV are described in the following sections (Tables 8.3 and 8.4 and Figs. 8.2, 8.3, and 8.4). In a recent RTOG 0615 trial, the lower-than-expected 2-year LRC rate of 84 % was attributed to an increased incidence of major deviations in target volume [19]. Thus, attention must be paid to accurate target delineation to avoid marginal misses when using IMRT.

Table 8.3
Definitions of target volumes for gross disease
Target volumes | Definition and description |
|---|---|
GTV70* (The subscript 70 denotes the radiation dose delivered) | Primary: All gross diseases on physical examination and imaging (see above regarding the importance of MRI) |
Neck nodes: All nodes ≥1 cm or those with necrotic center | |
CTV70* | GTV70 + ≥5 mm margin; around critical structures like the brainstem, 1 mm margin is acceptable |
PTV70* | CTV70 + 3–5 mm, depending on comfort level of daily patient positioning. Around critical structures like the brainstem, 1 mm margin is acceptable |
Table 8.4
Definition of target volumes for high-risk subclinical region
Target volumes | Definition and description |
|---|---|
CTV59.4* | CTV59.4 should encompass CTV70 with a 5-mm margin and regions at risk for microscopic disease which include |
Entire nasopharynx | |
Anterior 1/2 or 2/3 of the clivus (entire clivus, if involved) | |
Skull base (ensuring coverage of foramen ovale where V3 resides) | |
Pterygoid fossa | |
Parapharyngeal space | |
Inferior sphenoid sinus (entire sphenoid sinus in T3-T4 disease) | |
Posterior 1/3 of the nasal cavity/maxillary sinuses (ensuring coverage of pterygopalatine fossae where V2 resides) | |
Inferior soft palate | |
Retropharyngeal lymph nodes | |
Retrostyloid space | |
Bilateral nodal levels IB through V** | |
Include cavernous sinus for advanced T3-T4 lesions | |
Importance of reviewing bone window while contouring on CT scan to ensure coverage of skull base foramina | |
PTV59.4* | CTV59.4 + 3–5 mm, depending on the comfort of physician, but around critical structures like the brainstem, 1-mm margin is acceptable |

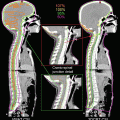
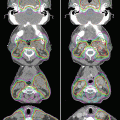
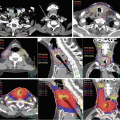
Fig. 8.2




Delineation of target volumes in a case of T1N1 nasopharyngeal carcinoma (NPC). GTV70 (inner contour, red) and CTV59.4 (green) contours in a patient with T1N1 NPC with coverage of the retropharyngeal and level II nodes (Figure 1.2 from: Lee NY, Le QT, O’Sullivan B, Lu JJ (2003) Chapter 1 Nasopharyngeal Carcinoma. Target Volume Delineation and Field Setup: A Practical Guide for Conformal and Intensity-Modulated Radiation Therapy, with kind permission from Springer Science + Business Media)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree