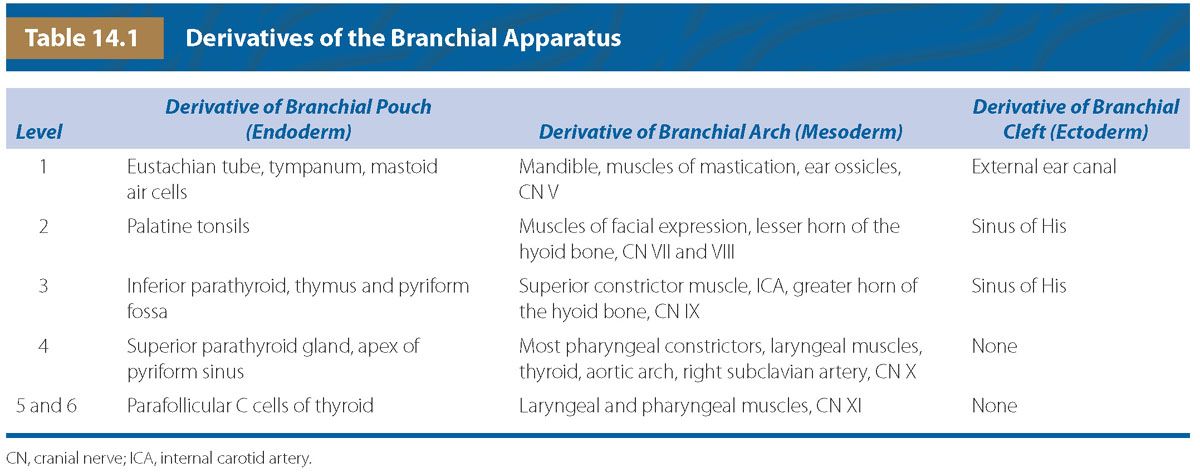
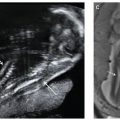
FIGURE 14.1: A: Differentiation of the pharyngeal clefts and pouches. There is elongation and growth of the second and fifth arches forming the sinus of His. B: Further maturation of the epithelium in the walls of the pharyngeal pouches. (Adapted from Benson MT, Dalen K, Mancuso AA, et al. Congenital anomalies of the branchial apparatus: embryology and pathologic anatomy. Radiographics. 1992;12(5):943–960.)
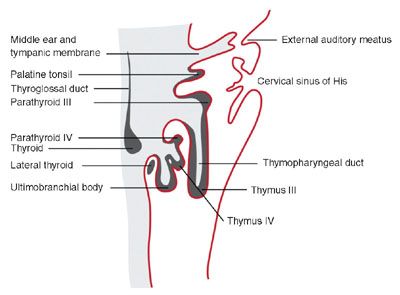
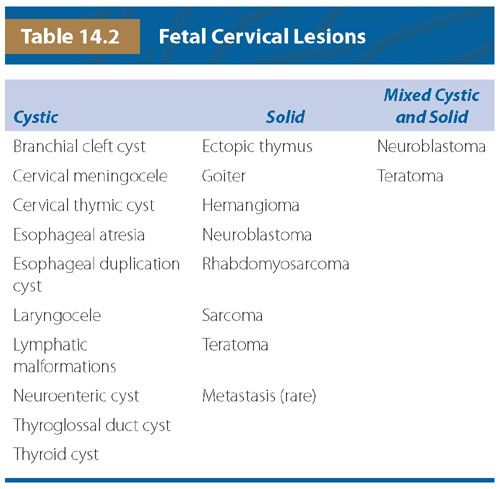
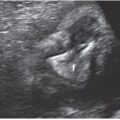
FIGURE 14.2: The structures that develop from the branchial clefts and pouches, particularly origin of the thymus, parathyroids, and ultimobranchial body. (Adapted from Benson MT, Dalen K, Mancuso AA, et al. Congenital anomalies of the branchial apparatus: embryology and pathologic anatomy. Radiographics. 1992;12(5):943–960.)
There are four ectoderm-lined clefts along the arches. In the 5th to 6th week, the second arch grows and elongates inferiorly to meet the enlarging fifth arch or developing epipericardial ridge (see Fig. 14.1A). As a result, an ectoderm-lined cavity forms enclosing the second, third, and fourth branchial clefts. This temporary cavity, known as the “Sinus of His,” usually obliterates secondary to fusion of its walls. However, failure of complete wall fusion can result in branchial cleft cyst, sinus, or fistula and, depending on the area of the branchial arch, can be classified into type 1, 2, 3, or 4 (see Fig. 14.1B). The first branchial is the only cleft that will become a definitive structure, eventually giving rise to the epithelium of the external auditory canal.
There are five pharyngeal or branchial pouches lined by endoderm, though the fifth evolves late and is usually considered part of the fourth. The first pouch gives rise to the pharyngotympanic tube that develops into the middle ear cavity and tympanic membrane (see Fig. 14.2). The second becomes the palatine tonsil. The third and fourth branchial pouches give rise to the thymus, parathyroid glands, and ultimobranchial body, representing parafollicular calcitonin secreting cells that are incorporated into the thyroid gland. With branchial pouch maldevelopment, there is lack of or erroneous formation of structures to which each pouch contributes. For example, incomplete obliteration of the thymopharyngeal duct may result in thymopharyngeal duct cyst or ectopic thymus, persisting along the tract of the migrating tissue (Fig. 14.3).
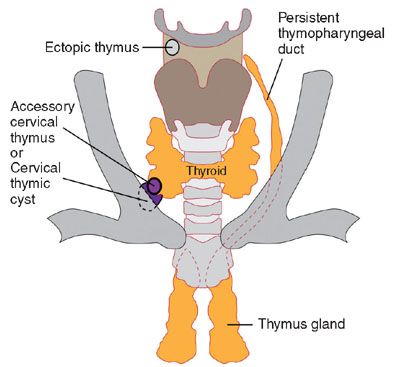
FIGURE 14.3: The course of the thymopharyngeal duct and possible locations for ectopic thymic tissue and/or thymic cyst. (Adapted from Benson MT, Dalen K, Mancuso AA, et al. Congenital anomalies of the branchial apparatus: embryology and pathologic anatomy. Radiographics. 1992;12(5):943–960.)
Table 14.1 summarizes the different structures arising from the branchial arches, clefts, and pouches. First arch syndrome includes anomalies secondary to abnormal development or absence of various components of the first pharyngeal arch. Examples include Treacher Collins Syndrome and Pierre Robin sequence.1
The thyroid gland arises during the 4th week as a small mass of proliferating endoderm at the apex of the foramen cecum in the developing tongue. It descends as a bilobed structure in the soft tissues of the neck attached to the thyroglossal duct that extends from the tongue, anterior to the hyoid and laryngeal cartilage to the lower neck. The thyroid reaches its final location in front of the trachea by the 7th week. At this time, the thyroglossal duct involutes, although if any portion persists, a thyroglossal cyst or sinus may remain. Infrequently, ectopic thyroid tissue can be found in the path of descent.
The lymphatic sacs begin to develop at the end of the 5th week of gestation, approximately 2 weeks after the cardiovascular system. There are six primary lymphatic sacs: paired jugular lymphatic sacs, cisterna chili, retroperitoneal (mesenteric) lymph sac, and paired iliac lymph sacs that will eventually evolve into groups of lymph nodes (Fig. 14.4). Lymphatic sacs develop alongside vessels and later make connections with the venous system. Lymphatic malformations may evolve secondary to incomplete or inadequate venous connections with stasis of lymphatic fluid causing dilated lymphatic channels.
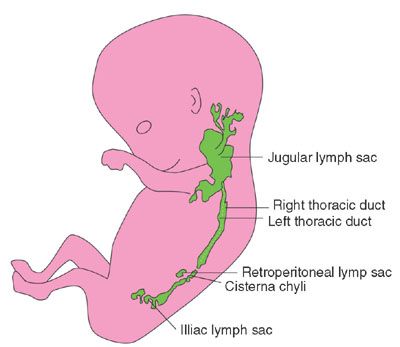
FIGURE 14.4: Seven-week embryo shows lymphatic sacs before the venous connections are formed.
CONGENITAL LESIONS OF THE NECK
Congenital neck lesions detected during the fetal period are very rare with unknown incidence. A list of congenital lesions of the neck is shown in Table 14.2.
Branchial Anomalies
Incidence: Prenatal diagnosis of branchial apparatus anomalies is very rare with few cases described in the literature.2–5
Pathogenesis/Etiology: Defects in the embryogenesis of the branchial apparatus include branchial, parathyroid, and thymic anomalies, which may manifest as sinuses, fistulas, or cysts. These anomalies can be further classified according to their branchial pouch or cleft of origin; branchial “cleft” cyst or branchial “pouch” cyst. Histologic evaluation of the epithelium lining the fistulas, sinuses, or cysts can define the pouch or cleft derivation.
The etiology of these lesions is poorly understood. The most accepted theory proposes that branchial anomalies are vestigial remnants resulting from incomplete obliteration of the branchial apparatus or buried epithelial cell rests.6 The most common lesion is a branchial cleft cyst type 2, which arises because of persistence of the cervical sinus of His. These lesions can be present in many anatomic locations, but the most common type 2 is a cyst in the anterolateral neck, most often on the left, lying anterior to the sternocleidomastoid, posterior to the submandibular gland, and lateral to the carotid sheath.2
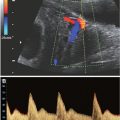
Diagnosis: By ultrasound (US), branchial cysts are round or ovoid anechoic or hypoechoic thin-walled cysts (Fig. 14.5). Real-time imaging can demonstrate movement of internal echoes differentiating from a solid lesion. On MRI, the cysts are hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences (Fig. 14.6).
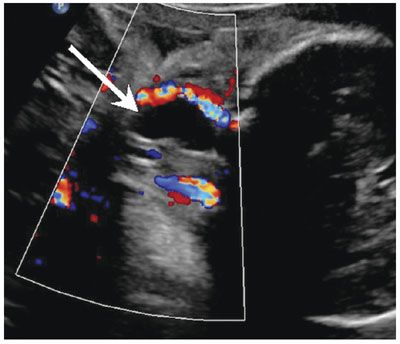
FIGURE 14.5: Branchial Cyst. Coronal Doppler US image shows an anechoic avascular cervical lesion (arrow) with acoustic enhancement in the lateral neck diagnosed as branchial cyst postnatal.
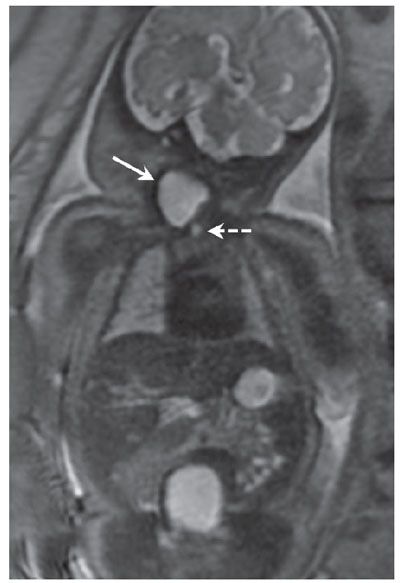
FIGURE 14.6: Branchial Cyst. Same fetus as in Figure 14.5. Coronal SSFSE T2 image shows hyperintense lesion (solid arrow) in the lateral fetal neck. The airway is partially visualized in this image (dotted arrow).
Associated Anomalies: Branchial cleft cysts are usually isolated lesions; however, they have been reported with branchio-oto-renal syndrome.7
Differential Diagnosis: Differential diagnosis includes thymic cyst, lymphatic malformation, and thyroglossal duct cyst, though branchial cysts are typically lateral, whereas thyroglossal duct lesions are predominately midline.
Prognosis/Management: Rarely, branchial cleft cyst can cause airway or esophageal obstruction and necessitate an ex utero intrapartum treatment (EXIT).2 Given susceptibility to hemorrhage, infection, and questionable predisposition to cancer, surgical resection, and marsupialization of the tract is curative. The prognosis is excellent following complete resection.
Cervical Thymic Cyst
Incidence: Thymic cysts are very rare and infrequently diagnosed in utero.
Pathogenesis/Etiology: Thymic cysts may be encountered in the neck, thoracic inlet, or mediastinum because of persistence of the thymopharyngeal tracts.8 The thymus is derived from the ventral division of the paired third and fourth pharyngeal pouches around 6 weeks of gestation. The thymic buds at each side migrate inferiorly, forming the thymopharyngeal ducts, extending from the angle of the mandible, along the carotid sheath to the superior mediastinum owing to attachment to the pericardium. During the 7th to 9th week, there is obliteration of the proximal ducts that then separate from the pharynx. Epithelial proliferation within the distal ducts gives rise to bilateral thymic tissue that fuse in the anterior mediastinum. Rests of thymic tissue, known as ectopic thymus, can be found along the normal path of descent.9
Diagnosis: Cervical thymic cysts are commonly multilocular, but may be unilocular and vary in size from 1.4 to 8 cm. They are intimately associated with the carotid space, splaying the carotid artery and jugular vein.9 Thymic cysts are more common on the left, and up to 50% have a mediastinal connection. By US, they are anechoic but may have internal debris (Fig. 14.7). On MRI, the lesions are hypointense on T1-weighted images and hyperintense on T2 (Fig. 14.8). If they are complicated by hemorrhage, the internal signal will be heterogeneous.10
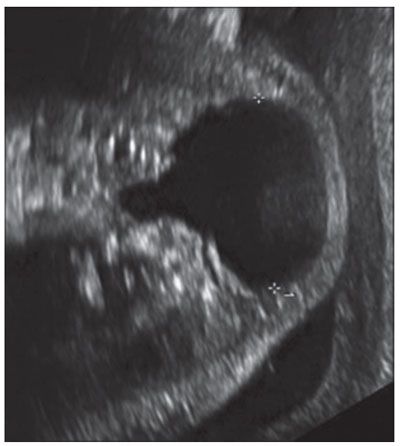
FIGURE 14.7: Thymic Cyst. Axial US image demonstrates lobulated anechoic lesion designated by calipers centered in the lateral neck diagnosed as thymic cyst postnatal.
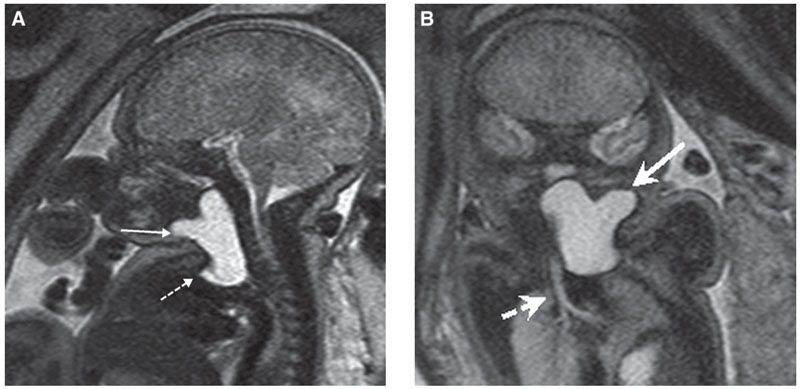
FIGURE 14.8: Thymic Cyst. A: Sagittal T2 SSFSE image of same fetus in Figure 14.7. Lobular lesion is T2 hyperintense (solid arrow) and extends the length of neck but is also present in the mediastinum (dotted arrow), contiguous with thymic tissue. B: Coronal T2 SSFSE image in the same fetus demonstrates lesion (solid arrow) and patency of the airway (dotted arrow).
Differential Diagnosis: The differential diagnoses include lymphatic malformation, thyroid cyst, thyroglossal duct cyst, and branchial cleft cyst.
Prognosis/Management: Thymic cysts are usually asymptomatic, although respiratory distress, dysphagia, and vocal cord paralysis have been described.11 Treatment consists of surgical excision of the cyst and residual tract although spontaneous resolution has been reported.11
Ranula
Incidence: The incidence of congenital ranula is estimated to be 0.7%. Prenatal diagnosis is very rare.12
Pathogenesis/Etiology: A ranula is a sublingual or minor salivary gland retention cyst located at the floor of the mouth. They are classified by location into simple (intraoral) or plunging (oral/cervical) types. Congenital ranulas are thought to arise secondary to atresia of the salivary gland ducts or ostial adhesion.12 The histopathology is distinctive with salivary duct epithelium lining the walls of the cyst, distinguishing from lymphatic malformation.
Diagnosis: By US, ranulas are well-defined anechoic or hypoechoic cysts in the floor of the mouth that may extend into the submandibular/cervical region (Fig. 14.9). No Color Doppler is usually observed. Well-delineated borders are seen on MRI with low T1 and high T2 signals (Fig. 14.10).
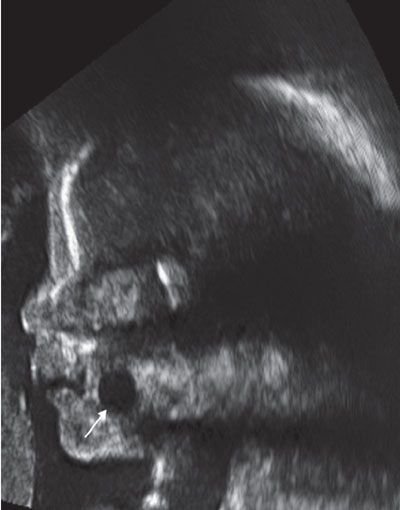
FIGURE 14.9: Ranula. Sagittal gray-scale US image demonstrates a round anechoic lesion (arrow) in the floor of the mouth, noted to represent ranula postnatal.
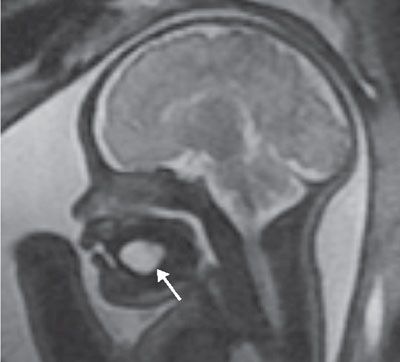
FIGURE 14.10: Ranula. Sagittal T2 SSFSE image shows a T2 hyperintense cystic lesion (arrow) in the floor of the mouth, below the tongue.
Differential Diagnosis: Differential diagnosis includes lymphatic malformation, thyroglossal duct cyst, and dermoid because of its midline/paramidline positioning.
Prognosis/Management: Congenital ranulas may present as cystic structures in the oral cavity and can rarely grow to completely fill the oral cavity, possibly obstructing the upper airway at birth.13 They elevate the floor of the mouth and cause the tongue to be superiorly and anteriorly displaced. Infrequently, giant congenital ranula may result in airway obstruction, necessitating EXIT procedure at birth to secure the airway.12,13 Treatments of congenital ranula include marsupialization, aspiration of the ranula, and resection of the sublingual salivary gland, all of which showed no recurrence except for marsupialization with 61% recurrence.14 Spontaneous resolution in the neonatal period has also been described.12
Thyroglossal Duct Cyst
Incidence: Prenatal diagnosis of thyroglossal duct cyst is rare.15 However, the cysts are the most common midline cervical anomaly, representing 70% of all congenital neck masses.16 The exact incidence is unknown, but 7% of the population has been shown to have a thyroglossal duct remnant.16
Pathogenesis/Etiology: The anatomy of the thyroglossal duct follows the pathway of embryology from the foramen cecum of the tongue, along the anterior surface of the hyoid to the pyramidal lobe of the thyroid. Persistence of the duct results in cyst or sinus formation at any point of descent. The majority of duct remnants are midline or parasagittal, adjacent to the hyoid bone.17 Rarely, these cysts are found within the tongue or the floor of the mouth.15 The lesion can be occasionally associated with Cowden syndrome.
Diagnosis: On US, the classic appearance of a thyroglossal duct cyst is a thin-walled anechoic unilocular lesion. However, because of high protein content, these lesions may be hypoechoic or heterogeneous echotexture, some appearing pseudosolid and mimicking ectopic tissue.18 Posterior acoustic enhancement is present in the majority of lesions, and absence of color Doppler flow can be helpful. Demonstration of a normal thyroid gland is recommended to exclude the diagnosis of ectopic tissue. On MRI, signal depends on protein content, but most are typically bright on T1 and T2 sequences.19
Differential Diagnosis: Other cystic lesions in the midline/paramidline include dermoid, esophageal duplication cyst, or ranula. Branchial and thymic cysts tend to be lateral neck. Lymphatic malformations and teratomas are typically complex cystic and solid.
Prognosis/Management: Up to one-third of patients with thyroglossal duct cyst will develop superimposed infection.18 There is a 1% increased risk of cancer, primarily of the papillary type.20 Rarely, the cyst may be within the tongue or floor of the mouth and can cause airway obstruction.15 In the presence of large cyst obstructing airway, an EXIT procedure may be necessary. Postnatal therapy is surgical resection with the Sistrunk procedure, which involves excision of cyst, remnant tract, and a portion of the hyoid bone.20
Dermoid/Epidermoid
Incidence: Prenatal diagnosis is rare, but overall these lesions represent 7% of head and neck lesions and 25% of midline cervical anomalies.20
Pathogenesis/Etiology: Dermoid/epidermoids develop when there is inclusion of ectodermal tissue during the fusion of the branchial arches. Dermoid cysts contain two germ layers, both ectoderm and mesoderm, whereas epidermoid cysts consist of only one germ layer, the ectoderm.21 Although the majority of these masses are found around the orbit or adjacent to the nose, approximately 11% of these cysts will present in the midline in the floor of the mouth in the submandibular space.20 These lesions can be associated with Gardner syndrome.19
Diagnosis: US of these lesions demonstrate a well-circumscribed, thin-walled unilocular mass with internal echos and little posterior echo enhancement. MRI depicts these lesions as T1 hyperintense to isointense, T2 hyperintense, and with a few lesions having fat–fluid or fluid–fluid levels. These are the only cystic lesions to restrict diffusion, which can confirm diagnosis.19
Prognosis/Management: Rarely, lesions in the floor of the mouth may cause airway obstruction, and EXIT may be considered. Surgical resection is the treatment of choice since these anomalies are at risk for rupture or infection, and 5% will undergo malignant degeneration to squamous cell neoplasms.20
INFLAMMATORY LESIONS
Thyroid Goiter
Thyroid goiter manifests as diffuse enlargement of the thyroid gland and may be associated with decreased, increased, or normal (euthyroid) function.
Incidence: Fetal goiter is very rare and can present with fetal hypothyroidism or hyperthyroidism. Hypothyroid goiter is more common than hyperthyroid, with incidence of 1 in 3,000 to 4,000 births worldwide.22 The incidence of hyperthyroid goiter is unknown. Graves disease is seen in 1% of pregnant women, and 2% to 12% of those fetuses will develop hyper- or hypothyroidism in utero or in the neonatal period.
Pathogenesis/Etiology: Fetal thyroid function begins in the late first trimester, approximately by week 12.23 The fetal thyroid slowly increases in size until week 32, after which a rapid growth pattern ensues. Several published nomograms compare gestational age and biparietal diameter to thyroid gland size to aid in the diagnosis of thyroid gland enlargement24–26 (see Tables 30 and 31 in Appendix A1).
Fetal goiter associated with hypothyroidism is primarily due to transplacental passage of maternal antithyroid treatment drugs (propylthiouracil, metimazole) or antithyroid antibodies, maternal iodine deficiency, or congenital thyroid dyshormogenesis. Maternal iodine intoxication by seaweed and iodine-containing vitamins are rare causes. Maternal thyroid function can be normal despite fetal goiter and should not deter monitoring and treatment of fetal thyroid dysfunction. In the absence of maternal thyroid disease, fetal goiter is likely due to congenital dyshormonogenesis. If untreated in the first 3 months of life, congenital hypothyroidism can cause severe irreversible mental retardation with impairment in speech and hearing.
Fetal goiter associated with hyperthyroid goiter is secondary to transplacental passage of maternal thyroid-stimulating IgG antibody, usually in women with Graves disease. It is not detected in the fetus before 20 to 24 weeks’ gestational age as the fetal thyroid is not mature enough to respond to antibody stimulation.
Diagnosis: Maternal thyroid function tests, including antithyroid antibody titers, should be obtained. However, maternal thyroid status does not reflect fetal thyroid state, and, therefore, fetal blood sampling may be required. The definitive diagnosis of fetal thyroid dysfunction is made by cordocentesis, with a risk for fetal loss of 0.5% to 1.4%.27 Historically, amniotic fluid analysis of fetal thyroid function is considered unreliable as there is poor correlation between amniotic fluid thyroid hormone levels and fetal serum levels.28 Fetal blood will show elevated TSH and low thyroxine in cases of hypothyroidism and low TSH in cases of hyperthyroidism.
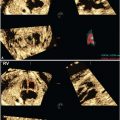
Ultrasound: Fetal goiter is diagnosed by US at an average gestational age of 26 weeks.29 The thyroid is symmetrically enlarged with bilobed configuration and varying echogenicity, either homogeneous or heterogeneous with multiple cysts. Enlargement of the thyroid may result in neck hyperextension seen via 2D and 3D ultrasound. The airway and esophagus may be compressed by the enlarged gland; polyhydramnios ensues if swallowing is impaired. Color Doppler shows increased vascular flow within the enlarged thyroid (Fig. 14.11). The pattern of increased central flow has been associated with hyperthyroid goiter, and peripherally increased flow with hypothyroid goiter.30 In fetuses with hyperthyroid goiter with high-output cardiac failure, Doppler may show increased velocities in the common carotid artery and descending aorta (∼200 cm per second) and dilatation of the superior vena cava. Cardiomegaly and pleural effusions have been reported.
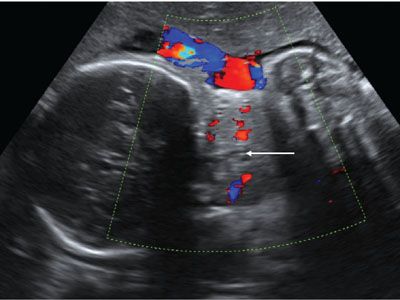
FIGURE 14.11: Thyroid Goiter. Coronal Doppler US image shows vascular enlargement of the thyroid. There is questionable mass effect on the airway (arrow).
In fetuses with hyperthyroid goiter, additional US findings include tachycardia, advanced bone age, hydrops, intrauterine growth restriction, and hepatosplenomegaly. In those with hypothyroid goiter, ultrasound may reveal cardiac dysfunction and delayed bone age.
MRI: On MRI, the normal thyroid gland is hyperintense on T1-weighted images and isointense on T2-weighted images, relative to muscle. With fetal goiter, there is symmetric thyroid enlargement with increased T1 and intermediate T2 signals31 (Fig. 14.12). Thyroid enlargement with T2 signal greater than muscle suggests thyroid dysfunction, either hypo- or hyperthyroid state. Intrinsic thyroid T1 hyperintensity persists despite hypo- or hyperthyroid state.32 MRI is useful in evaluation of tracheal and esophageal compression by the goiter as well as degree of neck hyperextension. MRI is particularly helpful in delivery planning as the most severe cases may require cesarean section or EXIT procedure to secure the airway at delivery. In this instance, MRI performed closer to delivery will best depict the airway.
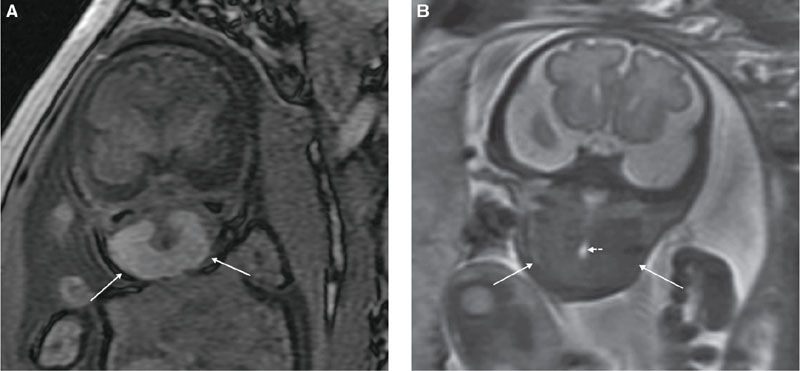
FIGURE 14.12: Thyroid Goiter. A: Coronal T1-weighted image demonstrates enlargement and hyperintensity of the thyroid gland (arrows). B: Coronal T2-weighted image shows isointense signal (with regard to muscle) of enlarged thyroid (solid arrows), which encircles narrowed but patent airway (dotted short arrow).
Differential Diagnosis: The differential diagnosis for fetal thyroid enlargement includes other fetal neck masses (cystic or solid), notably lymphatic malformation, hemangioma, and cervical teratoma. Cervical teratomas are usually midline, mixed cystic and solid, show rapid growth, and cause greater degree of mass effect upon the airway and esophagus and secondary polyhydramnios when compared with goiter. Lymphatic malformations are usually lateral rather than medial and are predominantly cystic. Hemangiomas characteristically have high vessel density and flow. Thyroglossal duct cyst, thyroid cyst, branchial cleft cyst, cervical neuroblastoma, cervical rhabdomyosarcoma, and ectopic thymus are much less common.
Associated Anomalies: Rarely, fetal goiter with hypothyroidism has been associated with other congenital anomalies, such as Prader–Willi syndrome.33
Prognosis:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree