Fig. 19.1
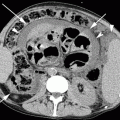
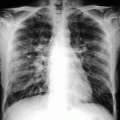
Obstructive pneumonitis. Posteroanterior chest film (a) in a 76-year-old man with cough and fever: pleural effusion and consolidation in the left lower lobe. Posteroanterior chest film 2 weeks after antibiotic therapy (b) no modifications on radiograph. Contrast-enhanced CT scan (c) shows pulmonary carcinoma causing left lower lobe bronchial obstruction, pleural effusion, bronchiectasis (arrow) with mucous plugging, and consolidation with dystrophic calcifications for obstructive pneumonitis (d)
When the chest radiograph raises the suspicion of malignancy (central or peripheral), the next consideration is represented by CT, especially with contrast medium, which is important both for diagnosis and for staging. The multidetector CT (MDCT) allows to perform tests in thin layers, with MPR, MIP, and minIP reconstructions, facilitating recognition of the characteristics of pulmonary nodules (diameter less than 3 cm), a peripheral mass (diameter greater than 3 cm), or a central mass compared to atelectasis (white triangular shape and absence of air bronchogram); the level of bronchi obstruction; the presence of obstructive pneumonia (presence of bronchi filled with mucus) (Fig. 19.2); any other parenchymal lesions; and a more accurate anatomical and morphological evaluation of lymph nodes (Muller et al. 2001).
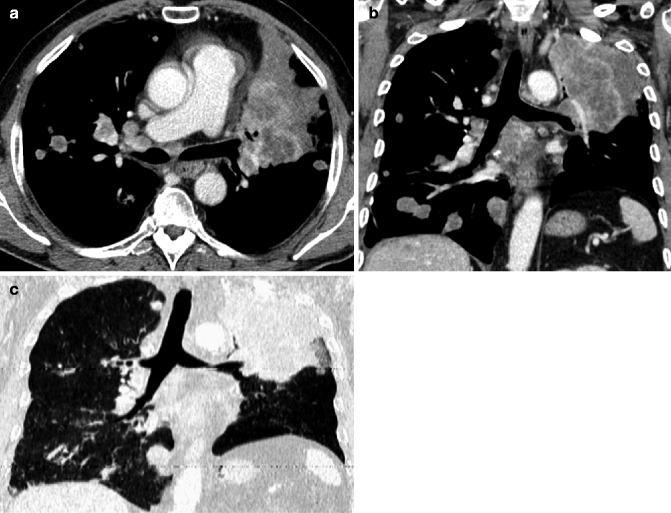

Fig. 19.2
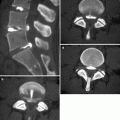
Axial contrast-enhanced CT scan (a), MPR coronal (b), and minIP coronal (c) reconstruction in a 66-year-old man with SCLC show the obstruction of the right upper lobe bronchus and the difference between the central mass and the atelectasis (*). Contralateral metastases are also demonstrated
The nodule on CT may appear with different densities: completely solid, partially solid and ground glass (Fig. 19.3), and completely ground glass (Tsubamoto et al. 2002) (Fig. 19.4). Its margins can be sharp, polycyclic, or spiculated. Net margins are usually a benign growth index (although in most cases, metastases occur in this way), while polycyclic margins and, above all, spiculated are almost always an expression of a malignant lesion (Fig. 19.5). The presence of calcifications can refer to malignancy of the nodule if in an eccentric position (Fig. 19.6), while their lamellar distribution, diffuse or central, suggests a benign lesion, usually of granulomatous type (Muller et al. 2001).
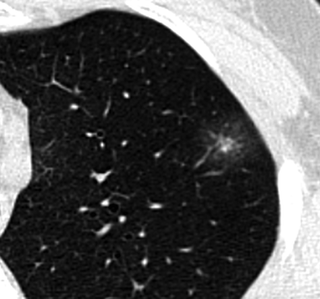
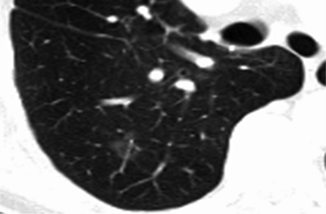
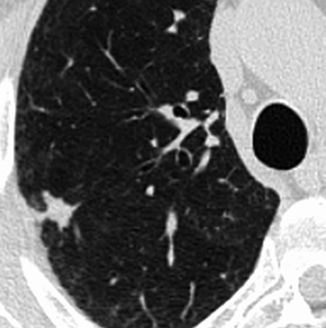
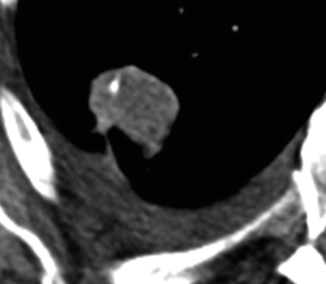

Fig. 19.3
HRCT shows a lesion in the left upper lobe in a 60-year-old man. The lesion is a mixed ground-glass nodule with a diameter of 18 mm, and a solid portion is present. The diagnosis of adenocarcinoma was proved surgically

Fig. 19.4
HRCT at level of right lower lobe in an 84-year-old man shows a 17-mm-diameter ground-glass nodule. The patient had a contralateral upper lobectomy for adenocarcinoma, and the diagnosis of a recurrent adenocarcinoma was proved by CT-guided percutaneous needle biopsy 2 years later (see Fig. 19.23)

Fig. 19.5
HRCT in a 72-year-old man shows a 2-cm-diameter nodule in the right upper lobe. The margins are lobulated, with some spiculations and thickening of adjacent visceral pleura. The diagnosis of adenocarcinoma was proved at surgery

Fig. 19.6
Calcification in pulmonary adenocarcinoma in a 72-year-old man. CT shows a 3-cm-diameter tumor in the right upper lobe with a focal area of eccentric calcification
With MDCT, it is possible to calculate the volume of the nodules, which is important for follow-up. Currently, there is no more reliable measurement of a solid node with the standard chest x-ray or CT scan; a three-dimensional technique is needed, with volume acquisition. It has been demonstrated that even subtle increases in the diameter corresponds to a significant increase in volume, an index of malignancy. An increase of about 26 % of the diameter corresponds to doubling of the volume, and doubling of the diameter corresponds to an eightfold increase in volume (Boll et al. 2004; Nair 2010) (Fig. 19.7). But we must also remember that if the nodule volume doubles in less than 7 days or more than a year, it is usually benign (doubling in a few days is usually indicative of inflammation) (Henschke et al. 2004).
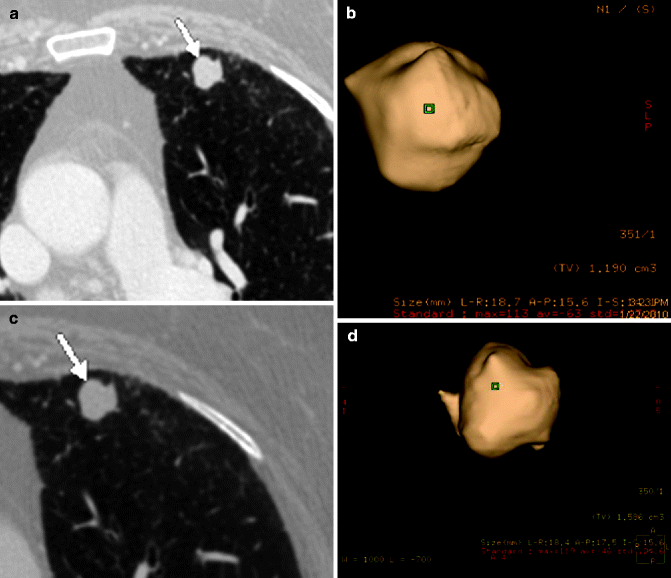

Fig. 19.7
CT scan of a nodule with polyciclyc margins (arrow) in the left upper lobe in a 73-year-old woman (a). Acquisition of volume (1,190 cm3) and diameters (18.7 × 15.6 × 13.4 cm) (b). Patient refused any other investigation. CT scan at the same level 8 months later (c) the nodule shows no apparent modification (arrow), but the volume is now 1,586 cm3 and the diameters are 18.4 × 15.5 × 15.6 cm (d). Patient again refused PET, biopsy, or surgery (Courtesy of Dr. Giulio Barbiero, Bassano del Grappa, Italy)
Finally, with MDCT it is possible to calculate the residual volume of the lung after surgery. This is very important in elderly patients because the association of this calculation with pulmonary function tests allows an accurate in vitro evaluation of the feasibility of surgery or another medical choice (Washko et al. 2010). Eighty percent of lung cancer are non-small cell lung cancer (NSCLC) (represented by adenocarcinoma, squamous cell carcinoma, and large cell carcinoma), and about 20 % are small cell lung cancer (SCLC) (Komaki et al. 2001).
19.2.1 Adenocarcinoma
Among the NSCLC, adenocarcinoma, which represents the most common form in young subjects, has an incidence of 30–35 % in the elderly, also affecting women, and those who have never smoked (Owonikoko et al. 2007). The majority of tumors present as a parenchymal nodule with a diameter equal to or less than 3 cm (Kishi et al. 2004) or, less frequently, as a mass with a diameter greater than 3 cm, also associated with lymph nodes. The adenocarcinoma is an epithelial type tumor characterized by the presence of neoplastic glands with variable production of mucus, forming berries or tubules, solid heaps, or growing along the alveolar walls (Gridelli et al. 2007).
It is almost always asymptomatic, and recognition is often by chance, on radiograms performed for other reasons, which is linked to the seat, the size, and the morphological characteristics (Aoki et al. 2001).
In 2011, the International Association for the Study of the Lung, the American Thoracic Society, and the European Respiratory Society proposed a new classification of this neoplasia, published in the Journal of Thoracic Oncology, to provide an integrated clinical, radiological, biomolecular, and anatomopathological approach (Travis et al. 2011). Also in light of the recent advances in understanding the disease, a unique terminology has been defined for the various subtypes of adenocarcinoma, with particular reference to the one previously classified as BAC.
It is well known that thin-slice CT is crucial in the study of pulmonary nodules that, in this histological type, may present with three different aspects, also in agreement with the Fleischner Society glossary (Hansell et al. 2008) and with the guidelines for sub-solid nodules (Godoy and Naidich 2009):
1.
Nodule with pure ground-glass appearance, as assumed to be a focal area of increased parenchymal attenuation, in the context of which the anatomical structures, such as lung bases, are easily identifiable
2.
Solid nodule, seen as a focal area of increased parenchymal attenuation, in which the normal anatomical components, such as lung bases, are no longer recognizable, with regular, spiculated, or polycyclic margins
3.
Partly solid nodule, seen as focal nodular opacity, both with a solid component and a ground-glass component
CT semiologic features are classified as follows, although there are overlaps in the various patterns of presentation and the follow-up direction of the various proposed histological subtypes:
AAH is a preinvasive lesion identified earlier on thin-slice CT. It appears as faint ground-glass nodules of small size (less than 5 mm), single or multiple, extremely slow growing (Fig. 19.8). The frequency and duration of follow-up are not unique because these nodules have a low probability of malignant transformation. In our view, this type of nodule does not require follow-up in elderly patients.
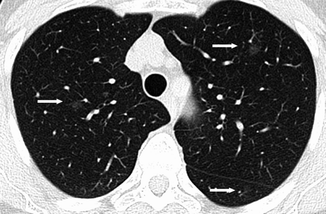
Fig. 19.8
AAH. Thin-section CT. Bilateral small (<5-mm-diameter) areas of pure ground-glass attenuation (arrows). Vessels and lung architecture are seen through the nodule
AIS is a noninvasive lesion, less than 3 cm, single or multiple, very slow growing (BAC corresponding in the previously used classification). The mucinous subtype typically presents as a nodule with a pure ground-glass appearance, with slightly higher attenuation than AAH (Fig. 19.9); it can also occur as a solid or partially solid nodule and can be “bubble-like.” The mucinous subtype appears as a solid nodule or as an area of consolidation.
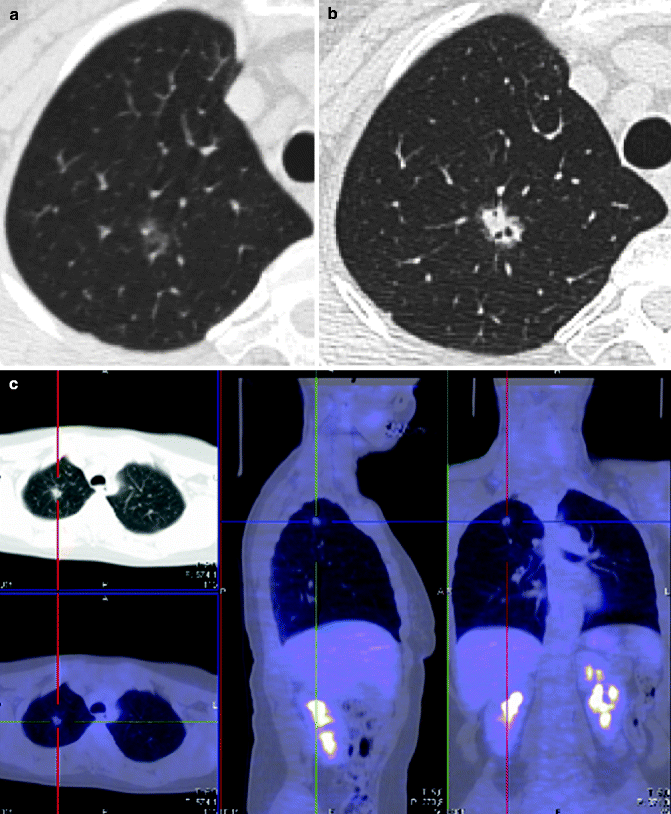
Fig. 19.9
Adenocarcinoma in situ in a 60-year-old woman with scleroderma. Thin-section CT (a) small (4-mm-diameter) areas of pure ground-glass attenuation in the right upper lobe. HRCT 4 years later (b) solid nodule 2.5-cm diameter with a bubble-like appearance. PET image (c) no activity of the lung nodule. The diagnosis was confirmed at surgery
MIA: the presentation can be variable. Usually the non-mucinous subtype presents as a partially solid nodule, with predominant ground-glass component and a solid central component of less than 5 mm.
The mucinous subtype may appear as a solid or partially solid nodule (Fig. 19.10).
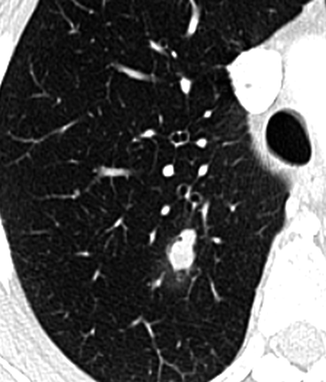

Fig. 19.10
MIA, mucinous subtype in a 71-year-old man. CT scan shows a solid nodule 1.3-cm diameter with polycyclic margins and halo sign in the right upper lobe. The diagnosis was confirmed at surgery
The size of the nodule correlates proportionally with vascular invasion and then with the metastatic spread of the disease, even at the central nervous system level.
In radiological practice, one must also bear in mind the following considerations:
Material obtained from biopsy must be sufficient for traditional microscopic analysis but also for immunohistochemistry and molecular analysis.
In thin-section CT, study of partially solid lesions is necessary to measure both the size of the solid component and the overall size of the lesion or of solid and ground-glass components.
Form, size, and degree of attenuation modifications can help determine the follow-up and appropriateness of surgery. It should be emphasized that in case of solid nodules, lesion stability after 2 years of follow-up is suggestive of a benign lesion and does not justify further testing.
Adenocarcinoma may develop on a preexisting pulmonary fibrosis, both in the form of a localized scar (ex-scar carcinoma) and idiopathic pulmonary fibrosis (Fig. 19.11). This is far rarer, since fibrosis, which is so common in lung adenocarcinoma, is the consequence of the action promoted by desmoplastic malignant cells rather than the cause of cancer (Balducci 2000). Regardless of the mode of growth, lung adenocarcinomas are tumors that frequently metastasize via lymphatics to regional lymph nodes (at least 50 % of cases) (Fig. 19.12) or by blood (20 % at diagnosis) to the liver, bone, central nervous system, and adrenal glands, or spread with neoplastic chips in the same lung or in the contralateral one (Parkin et al. 2002).
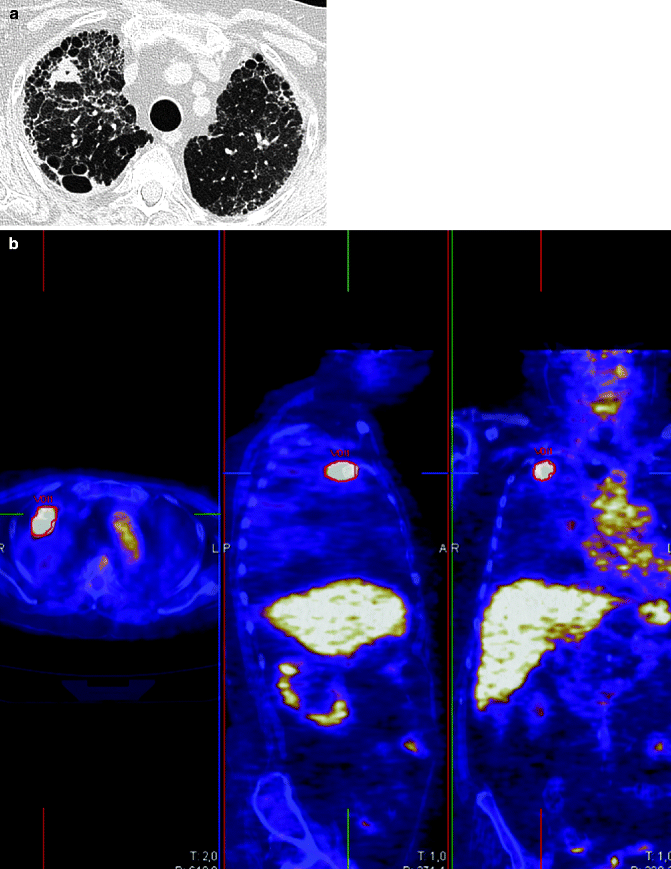
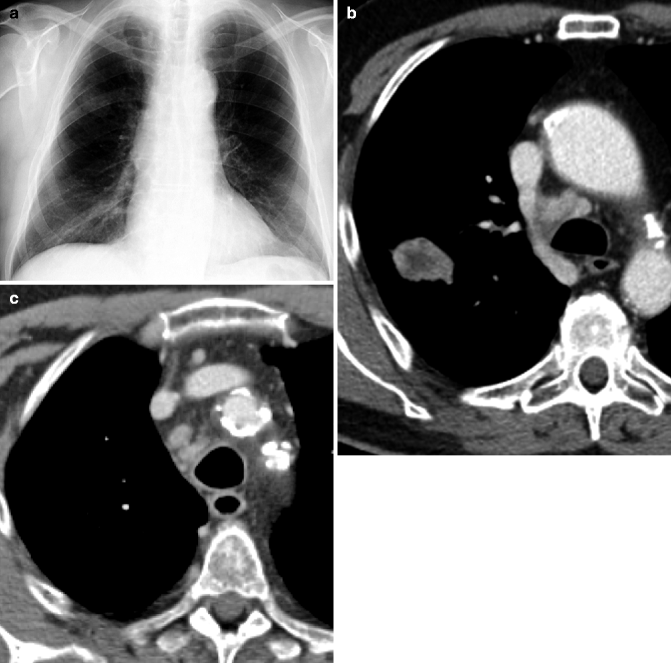
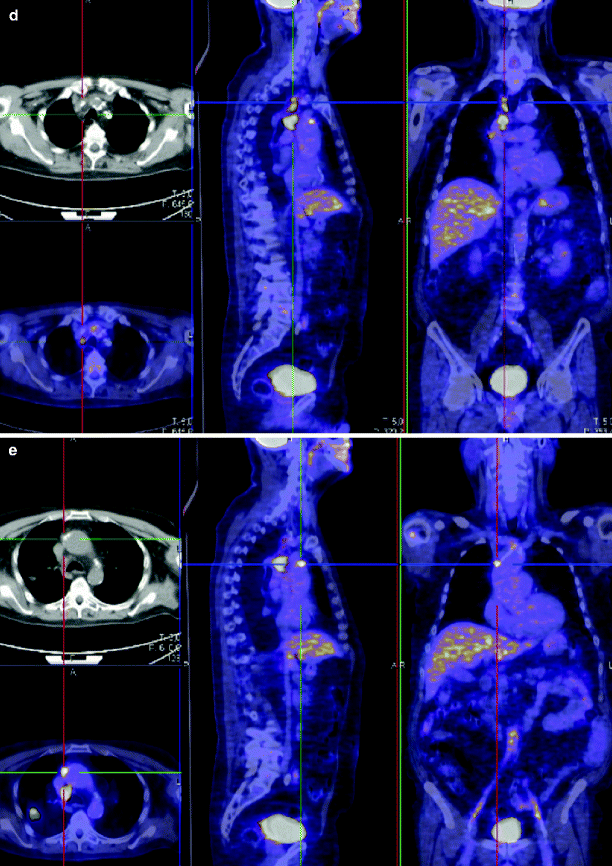

Fig. 19.11
Lung tumor and fibrosis in a 79-year-old woman. HRCT: pulmonary fibrosis with usual interstitial pneumonia pattern and 2-cm-diameter nodule with irregular margins and small cavitation in the right upper lobe (a). PET image (b) shows increased activity of the lung nodule (SUVmax 19.96)


Fig. 19.12
Lymph nodes metastasis. Chest film (a) and axial contrast-enhanced CT scan (b) in a 82-year-old man with 2.8-cm-diameter adenocarcinoma in the right upper lobe with right lower paratracheal lymph nodes and a 5-mm-diameter para-aortic lymph node (c). CT at level of epiaortic vessel shows right upper paratracheal 8-mm-diameter lymph node and highest mediastinal lymph node is 5-mm-diameter. PET-CT (d, e) confirms the presence of metastatic lymph nodes in all the stations
Especially in the elderly, one should take particular care in the differential diagnosis of a pulmonary nodule as this organ is one of the hardest hit locations in extrapulmonary metastatic spread of cancer. The frequency of a surgically confirmed metastasis in patients with no known history of other cancer is low (0.4–0.9 %), while if a lung nodule is recognized on CT in a patient with a known non-thoracic cancer, the probability of metastasis increases to 46 %, with a high possibility that it is a primary lung tumor (Quint et al. 2000).
Regarding the differential diagnosis of solid pulmonary nodules, benign lesions should be mentioned. In particular, should be excluded specific scarring lesions (often with calcifications inside or, sometimes, completely calcified), hamartomas (easily diagnosed by CT only if there are calcifications and/or fat component inside), and OP. This last, even if rarely, may manifest on CT as a single parenchymal lesion, frequently in the upper lobes and in contact with the pleura and fissures (pleural tags, in 38 % of cases) in a peripheral site and with a mainly oval or trapezoidal shape, with frequent presence of surrounding satellite lesions (Fig. 19.13). It can be recognized as an entering vessel or a small bronchus inside (Murphy et al. 1998; Polverosi et al. 2006).
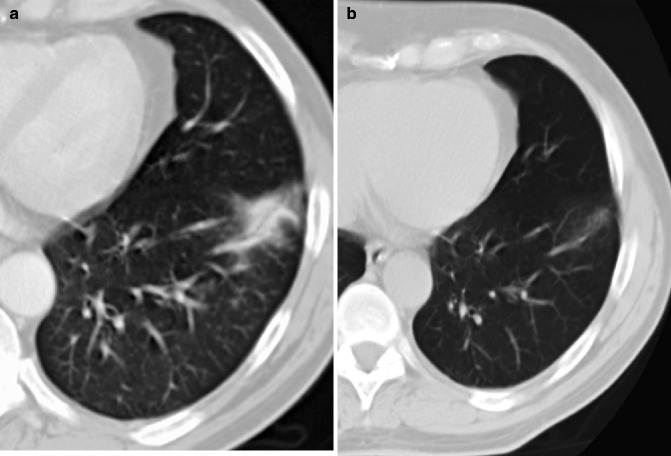

Fig. 19.13
OP. Thin-section CT. Consolidation of 4.5-cm diameter with small bronchi in the left lower lobe (a). The diagnosis was confirmed at bronchoscopy. CT after corticosteroid therapy (b) shows resolution of the lesion
19.2.2 Squamous Cell Carcinoma
Squamous cell carcinoma is more frequent in elderly men with a history of habitual cigarette smoking, with an incidence of 40–50 % (Gironés Sarrió et al. 2010), but it can be also in connection with the exposure to asbestos. This is confirmed by a study conducted by de Torres and collaborators (2011) that has shown that patients with COPD, gold stages I and II, male, elderly, with a low body mass index, and reduction of the DLCO below 80 % are the most frequently associated with the lung cancer, especially the squamous cell variant. Also other authors (Kondo et al. 2011) have confirmed the association between lung cancer, DLCO, male gender, and smoking in advanced age (however, not noting significant differences in the histotype neoplasm) in survival at 5 years and in mortality compared to patients without these special features (Fig. 19.14).
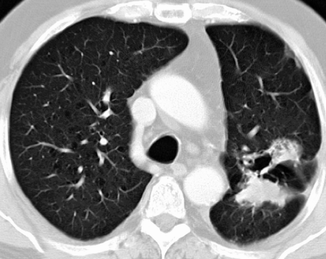

Fig. 19.14
Centrilobular emphysema and left upper lobe cavitated mass in a 61-year-old man who smokes. The diagnosis of squamous cell carcinoma was proved at bronchoscopy
About 70 % of squamous cell carcinomas develop as a hilar or perihilar lesion from the main proximal bronchi epithelium, causing atelectasis and obstructive pneumonia in lung distal parenchyma (Muller et al. 2001). Only 30 % are more peripheral lesions from the segmental, subsegmental, and terminal bronchi. A recent study, however, reported a prevalence of 53 % of peripheral squamous cell carcinomas (Fig. 19.15), suggesting a change in the epidemiology of this histotype, which probably develops from stem cells present in the basal layer of the bronchial elium (Owonikoko et al. 2007).
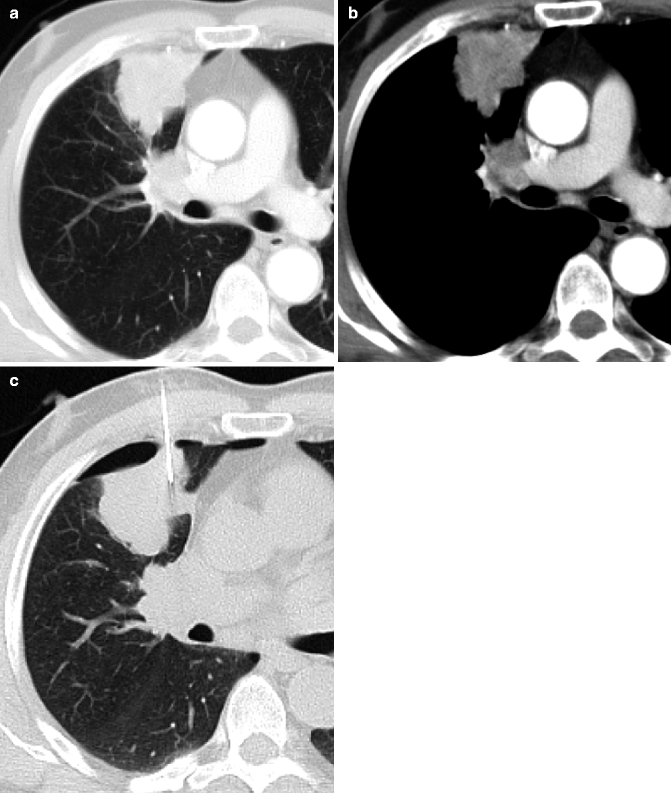

Fig. 19.15
Peripheral squamous cell carcinoma. CT scan shows a 7-cm-diameter mass in the anterior segment of the right upper lobe (a) with enlarged hilar lymph nodes (b) in an 80-year-old man. CT-guided percutaneous needle biopsy of the mass (c) showed squamous cell carcinoma (note pneumothorax also as a complication)
Most atelectasis and obstructive pneumonia is easily recognizable with chest radiography, but this loses specificity in the elderly as other diseases can result in a comparable framework from a radiologic point of view (think of aspiration pneumonia or atelectasis from a mucus plug in patients with bronchiectasis and/or COPD). The incidence of excavations in squamous cell carcinoma is about 22 % and almost always when the tumor has a diameter greater than 3 cm (Muller et al. 2001) (Fig. 19.16). This poses problems of differential diagnosis with lung abscess, inflammatory disease with an excavated central part, not uncommon in elderly patients with reduced immunity. The abscess, unlike an excavated tumor, is almost always associated with pleural effusion (sometimes also with empyema), and clinical conditions are typically worse in patients with inflammatory situation. The differential diagnosis may be more complicated when you have an overlap of inflammation in a peripheral tumor, especially if there is a central necrosis, with the formation of an abscess cancer, indistinguishable from a true inflammatory process.
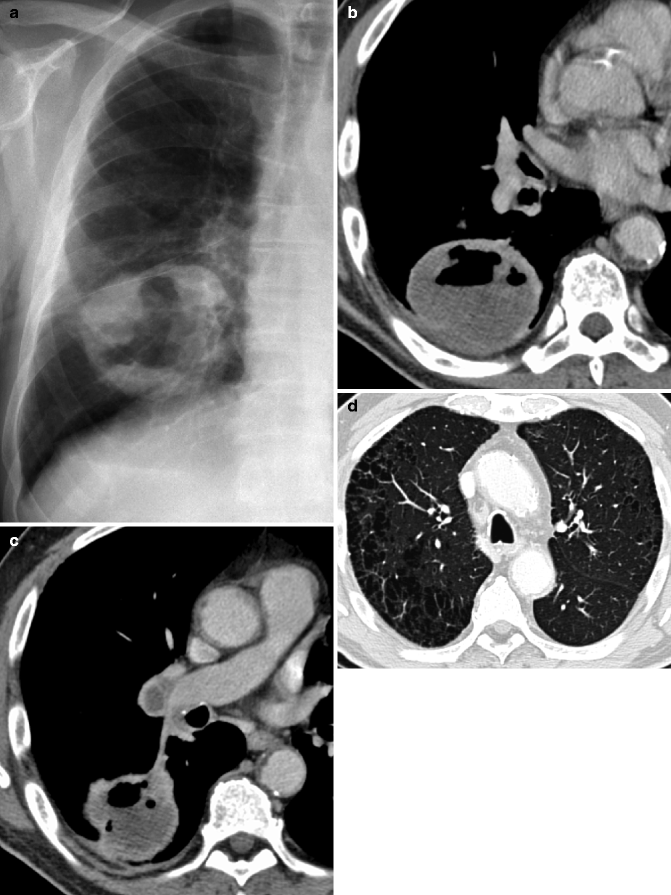

Fig. 19.16
Cavitary squamous cell carcinoma. Chest film (a) and contrast-enhanced CT scan in a 69-year-old man show a 5-cm-diameter mass in the right lower lobe, with an air-fluid level (b) and right necrotic hilar lymphadenopathy (c). CT also shows centrilobular paraseptal emphysema in the upper lobes caused by smoking tobacco (d)
19.2.3 Large Cell Carcinoma
Large cell carcinoma is responsible for 10–15 % of lung cancer, affects men with an average age of 60 years, and is largerly associated with smoking. They are a heterogeneous category of lung cancer, poorly differentiated by definition, of which there are several variants in common; they are difficult to differentiate and can suggest adenocarcinoma or squamous cell carcinoma (Muller et al. 2001).
They generally are peripheral lesions in variable relationship with bronchial structures.
The prognosis of large cell carcinoma of the classic type is generally worse than in common NSLC, also stratified by stage (Gridelli et al. 2007). Radiologically and by CT, it looks like a large mass (Fig. 19.17), with a frequency of excavation of about 6 % (Muller et al. 2001).
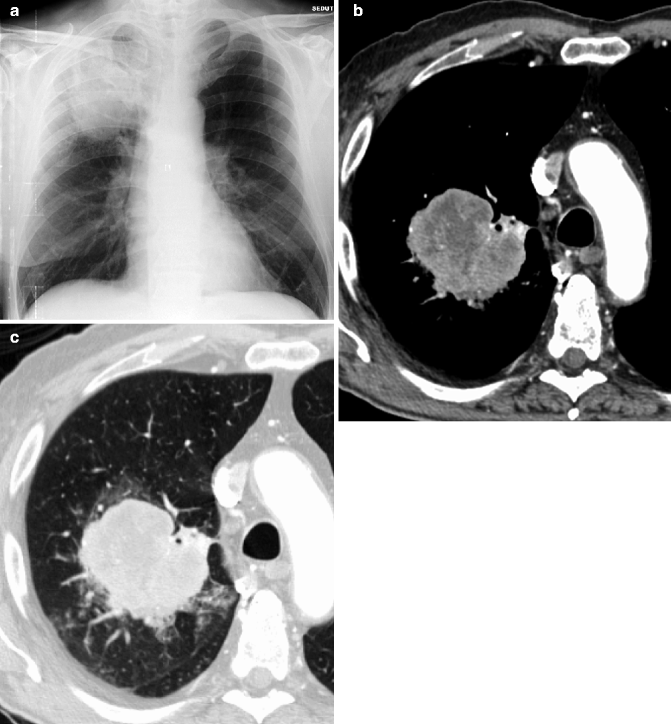

Fig. 19.17
Large cell carcinoma. Posteroanterior chest film (a) in a 62-year-old man shows a large mass in the right upper lobe. Contrast-enhanced CT scan with mediastinum (b) and lung window (c) demonstrates an 8.5-cm-diameter mass in the posterior segment of the right upper lobe, with an irregular interface and hypodense areas of colliquation. The diagnosis of large cell carcinoma was proved surgically
19.2.4 Small Cell Lung Cancer
The SCLC is one of the carcinomas with neuroendocrine differentiation, with acquisition of immunohistochemical and ultrastructural features similar to those found in cells of the diffuse neuroendocrine system (Carter 1983).
They represent at least 15–20 % of all lung cancers, and 60 % of these patients are over 60 years old (Muller et al. 2001; Simon and Wagne 2003). It is also associated with the habit of smoking tobacco. It is decreasing in recent years, rising from 17.4 % in 1986 to 13.8 % in 1998 (Simon and Wagne 2003), but tends to be much more aggressive than NSCLC, with an average survival in the absence of therapy between 2 and 4 months and between 9 and 18 months after therapy, depending on the stage in which the diagnosis is made (Weinmann et al. 2003).
The SCLC is 90 % central hilar-perihilar lesions in close association with the main bronchi which undermine the mucosa, filling up the bronchial wall to determine lumen stenosis without endobronchial growth. The involvement of lymph nodes, both hilar and mediastinal, is early and massive, but this happens less frequently in peripheral lesions that represent approximately 10 % (Muller et al. 2001). At diagnosis at least 70 % of small cell lung carcinoma presents with extrapulmonary metastases, massive involvement of mediastinal and contralateral lymph nodes, and metastasis to the contralateral lung (so-called extensive disease). Often there is pleural effusion, which can complicate the limited forms.
The chest radiograph, in typical form, shows a flare of mediastinal profile and a magnification of hila sometimes with mono- or bilateral pleural effusion. Contrast-enhanced CT confirms the presence of masses mainly in the hilar region, with narrow and compressed bronchi, but with no obstruction, associated with bulky mediastinal adenopathy, in the pretracheal region (even contralateral to the main mass), in the subcarinal region, and in the aortopulmonary window (Fig. 19.18). Pleural effusion is often present, and, in advanced stages with extensive disease, there are also nodular parenchymal lesions due to hematogenous spread and an interstitial reticular framework for carcinomatous lymphangitis. Infiltration in often observed from superior cava vein results in the appearance of superior cava vein obstruction syndrome.
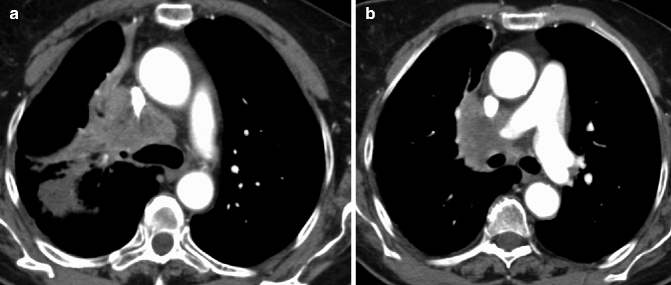

Fig. 19.18
SCLC in a 78-year-old man. Contrast-enhanced CT scan shows massive mediastinal and right hilar lymph nodes and airway compression (a) with invasion of the vena cava and right pulmonary artery (b)
The radiological pattern of SCLC poses problems of differential diagnosis with lymphoma, especially non-Hodgkin, frequently found disease in elderly patients, that may present with large hilo-mediastinal adenopathy.
19.3 Percutaneous Biopsy
The diagnostic conformation of a suspected lung tumor with radiological exams requires further investigation. Biopsy during flexible bronchoscopy (Hwangbo et al. 2010) and percutaneous biopsy are the techniques most widely used for providing an accurate cytologic or histological diagnosis of lung cancer. They should be considered as complementary procedures, although their position in the diagnostic algorithm remains a subject of debate.
Percutaneous biopsy (with CT guidance or, if anatomically possible, ultrasound guidance) is mainly indicated when the histological diagnosis can influence the therapeutic strategy or modify the staging of the disease and when the diagnosis cannot be established by bronchoscopic techniques (Laurent et al. 2003). The need for preoperative diagnosis of a pulmonary nodule that would obviate an unnecessary surgical thoracoscopy or thoracotomy is particularly important in older patients.
There is a general agreement that biopsy is indicated in patients who are inoperable because of tumor invasion, metastatic disease, or general condition and to determine the cell type in a lesion suspected of being either a metastasis, a second primary pulmonary carcinoma, or SCLC (Wu et al. 2011a). In view of the possible side effects (represented by hemorrhage and pneumothorax) (Fig. 19.19), this type of procedure must be carefully assessed before taking in an elderly patient (Wiener et al. 2011). Often they are chronically anticoagulated or have a coagulation disorder: abnormal clotting function or thrombocytopenia should be recognized and corrected before the procedure. Moreover, these patients almost always have several comorbidities.
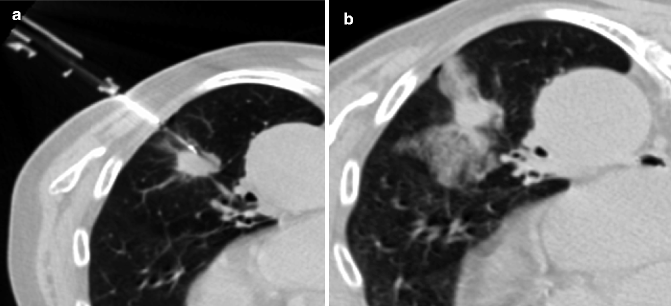

Fig. 19.19
CT-guided percutaneous needle biopsy of a nodule in the left lower lobe in a 73-year-old man (a). CT after biopsy shows consolidation and ground-glass opacities around the nodule consistent with hemorrhage (b). Patient had no symptoms and no treatment was required
Mechanical ventilation, which may lead to pneumothorax and favors air embolism, and inability of a patient to cooperate during the procedure or to suspend respiration on request or control cough are the major contraindications. A sole functional lung, severe chronic obstructive pulmonary disease, pulmonary hypertension, or cardiac insufficiency do not constitute absolute contraindications but render any complication more significantly (Wu et al. 2011b). In any case, the risks must be weighed against the benefit judiciously, and the availability of alternate procedures must be taken into account. CT fluoroscopy, for example, offers advantages of combining those of both fluoroscopy and CT, permitting biopsy of smaller lesions, lesions that are situated in less favorable locations such as those in the costophrenic recess or close to the mediastinum, and the procedure can be performed more rapidly in less cooperative patients (Wiener et al. 2011).
19.4 Follow-up of Pulmonary Nodules
If the chest radiographs show the presence of a small pulmonary nodule with a diameter less than or equal to 8 mm, later confirmed with MDCT, or if the nodule is directly detected with MDCT performed for other reasons, it is necessary to program the correct follow-up of the lesion to determine its benign or malignant nature (Hirsch et al. 2001). Patients considered at risk for pulmonary lung tumors are those over 40 (including the elderly), with a smoking habit, and familial history of cancer in first-degree relatives (Henschke et al. 2002).
To this, the characteristic of CT pulmonary nodules must be added: completely solid or ground-glass nodules, and presence of calcifications and smooth, speculated, or polycyclic margins are particularly suggestive for cancer. If MDCT characteristics of a solid pulmonary nodule suggest malignancy or if the diameter is greater than 8 mm, we can conduct further investigations to define the histological type and staging. Currently PET, especially if integrated with CT (PET-CT), is becoming increasingly important (Troung et al. 2010) (Fig. 19.20). On the contrary, if the lesion is nonspecific, it is appropriate to continue with radiological follow-up, considering age increases the risk of malignant lesions and sparing them from invasive procedures (bronchoscopy, biopsy).
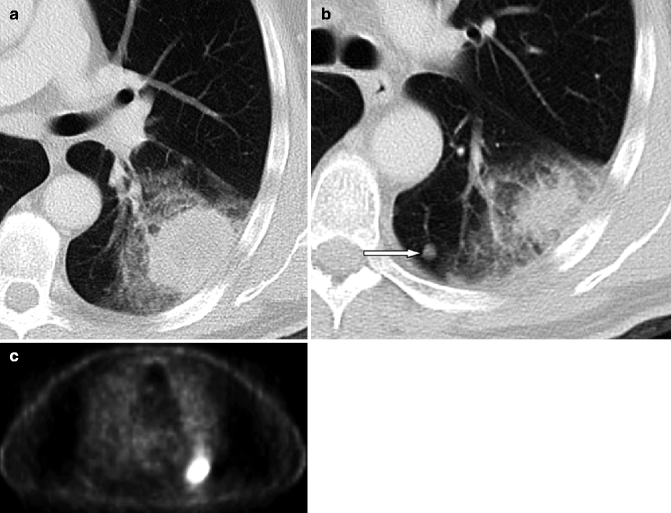

Fig. 19.20
Stage T3 according to TNM-7. A 72-year-old man with nodule in the same lobules as the primary tumor. CT scan shows a primary mass (a) with ground-glass halo and satellite nodule (b) in the left lower lobe (arrow). PET (c) shows radiotracer uptake in the mass but not in the nodule (false negative for 5-mm diameter). Surgery confirmed adenocarcinoma and a metastasis in the same lobes
The Fleischner Society (MacMahon et al. 2005) recommended management for solid pulmonary nodules in high-risk patients, relatively to the diameter:
1.
<4 mm → CT at 12 months; if unchanged, no further follow-up
2.
4–6 mm → CT at 6–12 months, then at 18–24 months if no change
3.
6–8 mm → CT at 3–6 months, then at 9–12 and 24 months if no change
4.
>8 mm → CT at 3–9 and 24 months, PET, and/or biopsy
Solid nodules with 8-mm diameter are considered potentially malignant. Either close CT follow-up or further investigations to immediately establish the nature of the nodule should be pursued.
In fact, the malignancy of a nodule is closely related to its size. Nodules with a diameter of less than 5 mm have a prevalence of malignancy of 0–1 %. When the diameter increases, there is a prevalence of 6–28 % for a diameter of 5–10 mm, 33–60 % for a diameter of 11–20 mm, and 64–82 % for a diameter larger than 20 mm (Wahidi et al. 2007).
So, for solid nodules with a diameter 8 mm–3 cm inclusive, recommendations for follow-up are more restrictive, suggesting immediate further examinations with other methods (PET-CT or biopsy) and direct surgical confirmation if CT features suggest the presence of a malignant lesion (Gould et al. 2007).
If the diameter of the nodule is 6–8 mm inclusive, with no sure features of malignancy in an elderly patient and without other risk factors (especially nonsmoker and without other tumors), CT follow-up at 3, 6, 12, and 24 months can be done to evaluate the stability of the volume of the nodule. It should be emphasized that in case of solid nodules, stability after 2 years of follow-up is suggestive of benign lesion and does not justify further investigations.
The situation is different for nodules greater than 1 cm in diameter with ground-glass aspect or partially solid and partially ground glass, where the prevalence of malignancy is, respectively, 18 and 63 % (Henschke et al. 2002; Godoy and Naidich 2009). So with nodules with these characteristics, the following behavior at follow-up has been suggested: in case of ground-glass nodules less than 1 cm in diameter, a CT follow-up is recommended after 3 months. If the nodule has regressed, no need for other controls. If it is stationary, CT follow-up should be scheduled for 6, 9, 12, and 24 months, but even if the nodule is stationary, the CT follow-up should continue because changes are slow in these nodules. The duration of follow-up is still unknown in this context.
If the nodule grows or dimensions are between 1 and 3 cm, both as pure ground glass and with mixed density (solid and ground glass), it is better to proceed directly with other methods (PET-CT, TFNAB, VATS) to exclude the presence of a malignant lesion (Oh et al. 2007; Goo et al. 2011) (Table 19.1).
Table 19.1
Follow-up of pulmonary nodules
Dimensions | Step 1 | Step 2 |
|---|---|---|
Solid nodules | ||
< 4 mm | CT 12 months | Stop (if unchanged) |
4–6 mm | CT 6, 12, 18 months | CT 24 months (if unchanged) |
6–8 mm | CT 3, 6, 12 months | CT 24 months (if unchanged) |
8 mm | CT 3, 6, 9 + PET-CT and/or biopsy | CT 24 months (if unchanged) |
8 mm–3 cm | PET-CT and/or biopsy | CT 24 months (if unchanged) |
Surgery | ||
Ground-glass nodules | ||
< 1 cm | CT 3 months | Stop (if regressed) |
CT 6, 9, 12, 24 months (if unchanged) | ||
1–3 cm | PET-CT, TFNAB, VATS | |
19.5 Staging
The seventh edition of the TNM staging manual (Table 19.2) was published in 2009 (Chansky et al. 2009) and makes important changes in the staging of lung cancers, including SCLC and bronchopulmonary carcinoid (extremely rare in the elderly) in the new system. These were previously defined in staging with a different classification. The need to update the manual on staging of lung cancer is related, as well as the ability to determine with greater accuracy treatment and prognosis, to ubiquitous use of MDCT, and, in recent years, to the introduction of new techniques, such as PET-CT and endoscopic ultrasound. Endoscopic ultrasound-guided fine-needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of mediastinal disease allow minimally invasive tumor staging (Murakami et al. 2012) and better assessment of the extent of disease, both at intrathoracic level and in the evaluation of the extrathoracic metastasis, with reduced invasiveness compared to VATS and mediastinoscopy. This accuracy in staging is very important for elderly patients who need an evaluation before choosing therapy (surgery, radiotherapy, or pharmacology) relative to baseline conditions and to the presence of concomitant disease, especially if this is possible with less invasive methods (Uybico et al. 2010).
Table 19.2
Seventh edition of the TNM staging system for lung cancer
Tumor | |
T1 | T1a: ≤2 cm |
T1b: >2 cm but ≤ 3 cm | |
T2 | T2a: >3 cm but ≤ 5 cm |
T2b: >5 cm but ≤ 7 cm | |
Endobronchial lesions more than 2 cm distal to the carina | |
Tumors that invade visceral pleura | |
Tumor causing atelectasis of less than entire lung | |
T3 | >7 cm |
Separate tumor nodules in the same lobe as the primary lesion | |
Endobronchial lesions less than 2 cm distal to the carina, but without involvement of the carina | |
Tumors with local invasion of the chest wall (including superior sulcus tumors), diaphragm, mediastinal pleura, and parietal pericardium | |
Tumor causing atelectasis of entire lung | |
T4 | Separate tumor nodules in the same lung but not in the same lobe as the primary lesion |
Tumors of any size that demonstrate local invasion of the mediastinum or carina, trachea, heart, great vessels, esophagus, or vertebral bodies | |
Lymph node | |
N0 | No involved lymph nodes |
N1 | Lymph nodes in the hilar, interlobar, lobar, segmental, and subsegmental regions |
N2 | Lymph nodes in the ipsilateral mediastinum including the upper paratracheal, prevascular and retrotracheal, lower paratracheal, subcarinal, paraesophageal, and pulmonary ligament regions |
N3 | Lymph nodes on the side opposite the primary tumor, and all significantly large lymph nodes in the ipsilateral or contralateral supraclavicular or scalene regions |
Metastasis | |
M0 | Metastatic disease absent |
M1a | Local thoracic metastatic disease including contralateral lung |
Malignant pericardial and pleural diseases | |
M2b | Distant or extrathoracic metastatic disease |
The main changes in the new classification include subclassifications T1 and T2 associated with tumor size; the change of T and M in the presence of another nodule in the same lobe, in the same lung, or in the contralateral lung; pleural involvement; and the introduction of the concept of intra- or extrathoracic metastasis (Kligerman and Abbott 2010; Nair et al. 2011). The differences between TNM-6 and TNM-7 are reported in Table 19.3. The staging of central tumors was unchanged.
Table 19.3
Differences between sixth and seventh editions of the TNM staging system for lung cancer
Sixth edition | Seventh edition | |
|---|---|---|
Tumor | ||
Size | Only a single size limit of 3 cm is used for differentiation between T1 and T2 tumors | New tumor size limits of 2, 3, 5, and 7 cm (to differentiate between stages T1a, T1b, T2a, T2b, and T3) |
Satellite nodule(s) in same lung and lobe as primary mass | T4 | T3 |
Satellite nodule(s) in same lung but not in same lobe as primary mass | M1 | T4 |
Lymph node | ||
Lymph node map | MD-ATS (Mountain-Dresler–American Thoracic Society) maps | IASLC proposed a new lymph node map |
Metastasis | ||
Metastatic disease | M0: absent | M0: absent |
M1: present | M1a: intrathoracic spread | |
M2b: extrathoracic spread | ||
Malignant pleural or pericardial effusion | T4 | M1a |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree