Introduction
Neurovascular spinal cord injuries are quite prevalent and in a busy trauma center radiology practice these injuries will be commonly seen. Magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and computed tomographic angiography (CTA) have greatly facilitated imaging neurovascular injuries. The introduction of MRI revolutionized the evaluation of neurovascular injuries associated with spinal trauma. Magnetic resonance imaging provides a very sensitive in-vivo analysis of the internal status of the spinal cord in trauma patients and MRI findings correlate well with the histopathological changes that occur secondary to acute spinal cord trauma.1–6 Patient prognosis has also been found to correlate with the MRI findings in spinal cord trauma.
Spinal cord infarcts due to arterial injury from trauma are relatively rare, but it has been shown by imaging that vertebral artery injuries are not an unusual occurrence. Magnetic resonance imaging and magnetic resonance angiography have dramatically facilitated the analysis of vascular injuries, particularly of the vertebral arteries, associated with spinal trauma due to their ability to demonstrate the patency of vessels and the presence of intramural hematomas in cases of dissection.7, 8 In addition, computed tomographic angiography (CTA) can provide information about the presence and extent of arterial injury.9 The vascular injuries that occur secondary to spinal trauma can lead to brainstem, cerebellar, and cerebral strokes.
Magnetic resonance imaging and MRA techniques are often the preferred procedures of choice for evaluating neurovascular injuries because of their proven accuracy and because they are noninvasive. Magnetic resonance imaging is better than conventional spinal radiography, myelography, and computed tomography with or without myelography for demonstrating the soft tissue injuries that occur in spinal trauma. Computed tomographic angiography has also been demonstrated to be useful for showing arterial injuries associated with spinal trauma. Conventional angiography, however, does remain quite useful for evaluating arterial injuries and several studies have shown that it is overall the best technique for demonstrating arterial injuries secondary to spinal trauma.
MR Imaging/Histopathological Correlation of Acute Spinal Cord Injury
Studies in human and animal models have thoroughly evaluated the pathological changes of traumatic spinal cord injuries.10–14 Edema, hemorrhage, mechanical axonal disruption, and severing of the nerve roots are the early pathological changes that occur as a result of trauma to the spinal cord.10–14 Traumatized nerve fibers undergo swelling, fragmentation, and disintegration of both axons and of myelin.10,14
Reversible and irreversible traumatic injuries to the spinal cord demonstrate pathophysiological differences.10 Less severe trauma causes reversible lesions and in these lesions there is mild vascular disturbance, inflammation of a minimal to moderate degree, and petechial hemorrhages that improve over time. Injuries caused by greater forces result in irreversible lesions in which the vascular system to the cord becomes compromised, and hemorrhages in the spinal cord coalesce and increase in extent over time.10 Inflammation and axonal swelling is seen early in the irreversible lesions and the changes become severe chronically (at 5 days) with extensive central hematomas and marked necrosis of the gray matter.10
Hyperacute traumatic spinal cord lesions in human autopsies rarely demonstrate hematomyelia. Hemorrhage usually does not occur in traumatic spinal cord injuries unless the patient survives more than a few hours.12 True hematomyelia was found in an autopsy series in only one of 123 patients with hyperacute spinal cord injury.12 The spinal cords were found either to be grossly intact or totally disrupted.12 However, the mere continuity of the gross specimen does not mean the nerve fibers have not suffered irreversible damage.12
The direct effects of the trauma itself are not the only mechanism of injury in spinal cord traumatic injuries. The presence of a secondary mechanism is suggested by the fact that the pathological changes worsen after injury.13 A secondary vascular mechanism in addition to the effects of the direct trauma likely leads to the development of post-traumatic ischemia and resultant infarction of the spinal cord.13 The injury to the spinal cord due to post-traumatic ischemia arises from systemic and local effects. Systemic effects of acute spinal cord injury are hypotension and reduced cardiac output while the local effects result in loss of autoregulation and a marked reduction of the microcirculation in both gray and white matter of the injured segment of the spinal cord.13,15 Oligodendrocyte apoptosis also occurs in the spinal cord after injury and evidence implicates this as a key determinant in the extent of neurological damage and dysfunction.16
The MRI findings correlate nicely with the histopathological findings in traumatic spinal cord lesions in animal models.17–21 Experimentally, however, producing only edematous spinal cord injuries, as are seen in humans, have been difficult.20 Histopathologically varying degrees of hemorrhage surrounded by edema in the spinal cord is seen in acute traumatic spinal injuries and MRI detects hemorrhage and edema acutely in the spinal cord that is traumatically injured.17–21 The microscopic abnormalities resulting from the trauma such as inflammation, nerve fiber swelling, nerve fiber tearing, apoptosis, and disintegration are not directly seen by MRI since MRI only provides a macroscopic evaluation of the spinal cord. Less severe injuries have been found to correlate with smaller abnormalities on MRI and more severe lesions have larger MRI abnormalities.17,18,20
MRI Features of Spinal Cord Hemorrhage
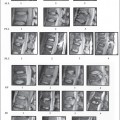
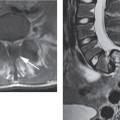
High-field MRI shows acute spinal cord hemorrhages, proven by pathological correlation, to appear hypointense on T2W images (Fig. 7.1a).20,21 The appearance and detection of blood products at lower field strengths (<0.5T), has been more variably reported than in series performed at higher field strengths. Acute hemorrhages at 0.5T have been reported to be hyperintense to hypointense on T2W images in the spinal cord.2,22–24 T2 hypointensity is more likely found in larger hemorrhages imaged at 0.5T.18 When a hemorrhage imaged at lower field strength appears hyperintense on T2W images the acute blood will have a signal intensity similar to edema making the hemorrhage inseparable from edema. High-field or low-field MRI usually will only show subtle signal intensity changes on T1W images in acute spinal cord hemorrhages and the signal intensity of the hemorrhage will often be isointense to the spinal cord and few cases are found with minimal hypointensity (Fig. 7.1b).6,18,21,22 High-field MRI will demonstrate blood products better than low-field MRI. However, the performance of gradient-echo imaging at lower and higher field strengths will help to detect blood products that may not otherwise be identified (Fig. 7.1c).
Spin-echo or fast spin-echo MRI techniques for imaging spinal trauma may be utilized and each technique has different advantages.25 Spin-echo techniques take longer to perform than fast spin-echo techniques but spin-echo techniques are more sensitive to blood products when compared to fast spin-echo techniques.25 However, the time saved by performing fast spin-echo MRI is so great that in general fast spin-echo MRI is the preferred technique for T2W spinal imaging. The time saved with fast spin-echo techniques can be spent to significantly increase the resolution of the scan and still have a shorter scan time. The weakness of fast spin-echo techniques for the demonstration of hemorrhage can be overcome by adding a gradientecho sequence and gradient-echo techniques are more sensitive than spin-echo MRI for detecting hemorrhage anyway.18,22,25 Unfortunately, the gradient-echo technique by itself is not suitable to be used as the only sequence for imaging spinal cord trauma since it is very poor at demonstrating spinal cord edema.25
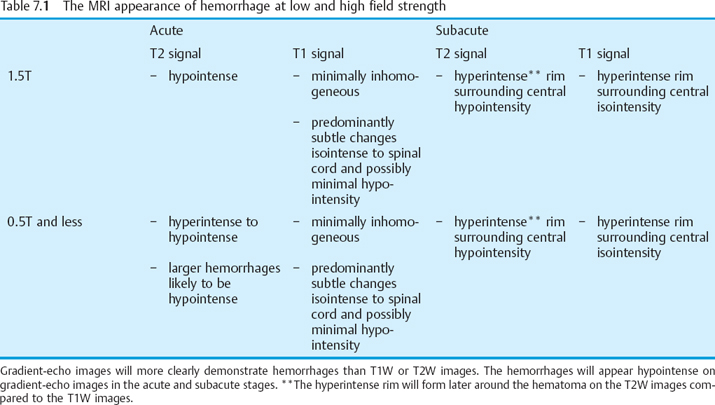
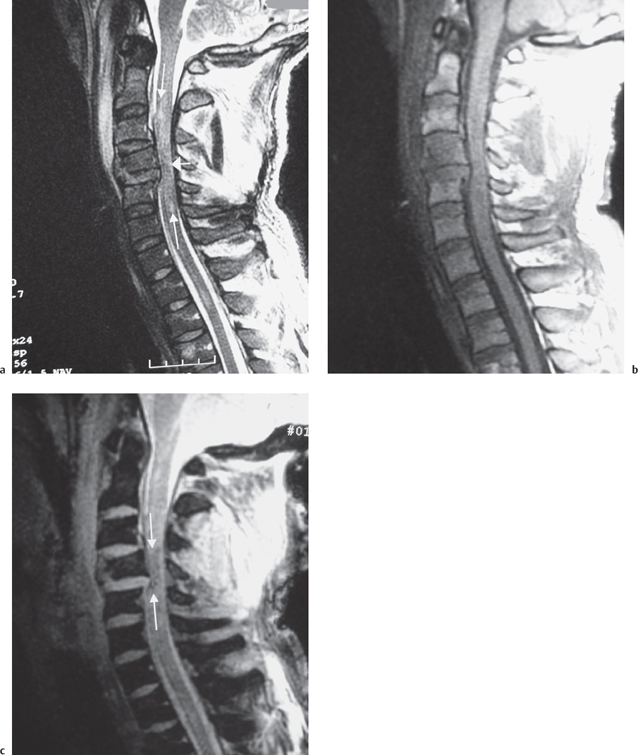
Fig. 7.1 28-year-old man status trailer falling on neck with resultant C4 vertebral body, facet, and lamina fractures. This examination was performed at 1.5T. a Sagittal T2W image demonstrates the C4 vertebral body fracture with mild retropulsion into the spinal canal. There is an extensive hyperintense signal consistent with edema in the spinal cord (long white arrows). An area of hypointense signal is present in the spinal cord presumably from hemorrhage (short white arrow). b Sagittal T1W image demonstrates at most minimal hypointensity at the area of injury. c A sagittal gradient-echo image demonstrates the hemorrhage as hypointense in the spinal cord (arrows). Extensive prevertebral soft tissue swelling is present in the mid and upper cervical spine.
In the acute setting hemorrhage in the spinal cord is deoxyhemoglobin and appears as described above. With the passing of time hemorrhage in the spinal cord will progress to methemoglobin. The time frame by which hemorrhage in the spinal cord progresses to methemoglobin based upon the changing signal intensity of the blood has been reported to range from 3 to 10 days.5,6,24,26,27,30,31 Hematomas in the spinal cord in general will show signal intensity changes progressing from the rim of the hematoma to the center. The signal intensity change consists of the development of a hyperintense rim around the center, which appears isointense on T1W images and hypointense on T2W images.6,27–30 However, the T1W images will develop the ring of hyper intense signal in the hematoma prior to the T2W images. The earlier T1W change corresponds to the development of intracellular methemoglobin whereasonthe T2W images the hematoma will not become hyperintense until enough extracellular methemoglobin is formed (Table 7.1, p. 101).28,29
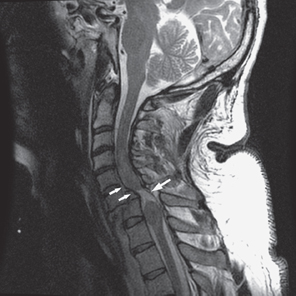
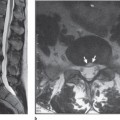
Fig. 7.2 46-year-old man status post motor vehicle rollover with bilateral jumped facets. A sagittal T2W image of the cervical spine demonstrates a 50% spondylolisthesis of C6-C7 with an associated spinal cord transection. Edema and spinal cord swelling surrounds the area of transection (large arrow). Behind C5 and C6 vertebral bodies there is a small epidural hematoma present (small arrows).
Prognostic Role of MR Imaging in Neurological Outcome
Spinal cord injury as demonstrated by MRI in humans’ in-vivo corresponds to abnormalities found in animal models of spinal cord trauma. The commonly occurring hemorrhage and edema in spinal cord trauma has been well documented by MRI, and MRI nicely demonstrates the rarely occurring cord transection (Fig. 7.2). Cord swelling is also found at the regions of injury on MRI examinations. Spinal cord compression from bone fragments, disk material, or hemorrhage is well demonstrated on MRI. A normal spinal cord may also be demonstrated on MRI in patients with neurological abnormalities.
The MRI appearance of the spinal cord in the setting of trauma can help predict the neurological outcome. Patients with normal appearing spinal cords uniformly have a good outcome, patients with only spinal cord edema do worse, and patients with hemorrhagic spinal cord injuries have even poorer neurological outcomes compared to patients with only edema. Patients with transected cords, of course, uniformly have the worst neurological outcomes. However, besides patients with transected cords, there is certainly variability seen in the neurological outcome between individual patients.
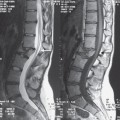
A classification of spinal cord injuries that has been found to be useful in characterizing injuries includes three patterns.6,27,30 Pattern type 1 is thought to represent hemorrhage, pattern type 2 is thought to represent edema (Fig. 7.3), and pattern type 3 is presumed to contain both edema and blood (Fig. 7.1).6,27,30 The type 1, hemorrhage pattern, correlates with severe neurological deficits and poor outcome.6,27,30 type 2 had the best outcome and type 3injury outcomes were variable.6,27,30
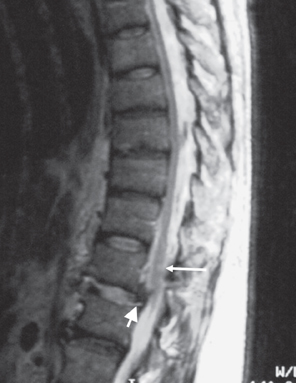
Fig. 7.3 48-year-old man who suffered a crush injury at work. He has a T12 on L1 fracture subluxation and was not moving his lower extremities at presentation. This is a sagittal T2W image that demonstrates a small area of hyperintense signal consistent with edema in the spinal cord at the T12 level (long arrow). There are several millimeters of spondylolisthesis of the T12 vertebral body compared to the L1 vertebral body (short arrow). There was excellent recovery of motor function and he was walking with a cane at 2 months after the injury.
Expanding the number of patterns of characterization of traumatic spinal cord injury to six has been proposed.31 The additional types of patterns include a normal pattern, a compression pattern, and a transection pattern. Patients with a normal spinal cord pattern do not have any neurological sequelae on follow-up.31 The compression pattern and especially the transection pattern indicate poor neurological outcome.31 However, the cord compression pattern, demonstrating severe obliteration of the spinal cord and significant alteration of cord morphology, did have a somewhat variable neurological outcome.31 With the cord compression pattern seven of nine patients described had complete spinal cord injuries and no change in clinical outcome, but two of the patients had only incomplete spinal cord injuries and favorable outcomes.31 Others report cord compression detected on the initial MRI or residual cord compression seen on MRI after reduction is associated with greater neurological compromise.5,23 Yamashita et al found that if the spinal cord had normal signal on T1W and T2W images, the patients had a good prognosis despite initially having severe cord compression.23 The presence of abnormal signal on T1W images indicates greater injury and a poor prognosis, although this study did not differentiate if the T1W changes were from hemorrhage or edema.23 Others have not found cord compression on the initial examination to be an important indicator of neurological outcome.22
The presence of hemorrhage in the spinal cord after spinal cord injury is associated with a significantly greater decrease of motor function than if there was no hemorrhage detected by MRI.32 Hemorrhage was never detected without edema in this study as has been previously described as a type 1 pattern.6,30 It is possible that no edema was detected because all the patients underwent MRI with a 1.5T scanner within 72 hours of injury. The edematous portion of an injury on studies that have a greater time delay to imaging may have time to resolve.32 Smaller hemorrhages may also have been detected due to improved spatial and contrast resolution of the study.32 Eighty-five percent of subjects with complete motor deficits had hemorrhagic lesions on their initial exams,32 but hemorrhage was not always associated with a complete motor deficit as has been previously described.5,33 In fact, 21% of incomplete motor injuries were found to have evidence of spinal cord hemorrhage on initial examination.32 Schaefer et al described a couple of small spinal cord hemorrhages in patients who exhibited limited improvement of their motor deficits.34
Neurological outcome has been associated with the extent of edema in the spinal cord after spinal cord injury.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree