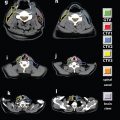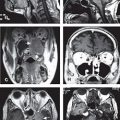
FIGURE 4-7. Differentiation of ECE (+) and ECE (−) necks in patients receiving postoperative IMRT. In the ECE (+) neck, soft tissues are more generously included, and the CTV1 and CTV2 should extend close to the skin surface, especially in the region or level of the ECE (−) node(s) specified by pathologic findings. ECE, extracapsular extension
1. INTRODUCTION
• Intensity-modulated radiation therapy (IMRT) provides greater dose conformality and is commonly used in the treatment for head and neck cancers.
• IMRT can protect critical normal tissue without compromising target coverage, but requires extensive knowledge of tumor spread and the ability to accurately delineate both the tumor target volumes and the cervical lymph nodes.
• Careful understanding of tumor spread ensures the delineation of the clinical target volume (CTV), which represents the region containing microscopic disease as defined in the International Commission on Radiation Units and Measurements Reports 50 and 62.1,2 In 2010 ICRU issued a new set of guidelines specifically for the use of IMRT, report number 83.3 This document should be read by all practicing radiation oncologists.
• Neither physical examination nor the radiologic imaging techniques used in clinical practice are sufficient in detecting microscopic disease. Sako et al.4 found that the submandibular nodes must measure at least 0.5 cm in size to be clinically detectable, while deep cervical nodes located adjacent to muscles must exceed 1 cm in diameter to be clinically palpable. Computed tomography (CT) is not sufficient to detect micrometastasis <1 cm in diameter.5
• The incidence of occult nodal metastasis ranged from 25% to 60% from the 1950s to the 1960s. Even with advances in morphology-based imaging techniques, such as helical CT or magnetic resonance imaging (MRI), determination of nodal metastasis on the basis of the size of the lymph node still underestimates between 12% and 60% of micrometastases. In addition, detection of micrometastasis by positron emission tomography (PET) has not demonstrated any further benefit, with sensitivities of 67% to 79% and specificities of 82% to 95%.6,7 Therefore, the current clinical practice to determine target volume for IMRT relies on historical information from surgical pathologic experiences.
• In 1948, Rouviere8 described the anatomic details of the cervical lymphatic network. On the basis of this description, the TNM atlas proposed a terminology that divides the head and neck lymph nodes into several groups according to their relationship with the adjacent muscles, vessels, and nerves.9
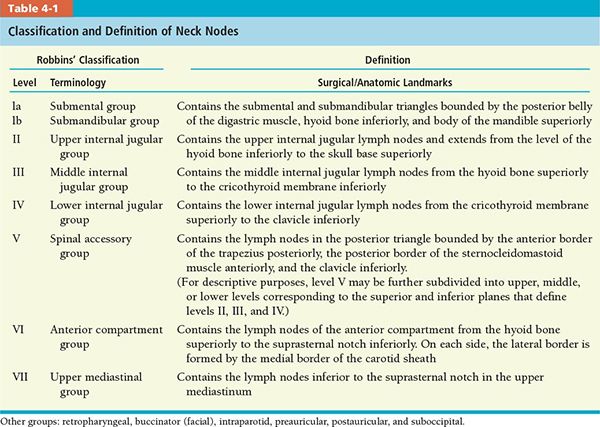
• In 1991, the American Head and Neck Society (AHNS) postulated a classification (Robbins’ classification) that divides the neck into six levels or eight nodal groups for those lymph nodes routinely removed during neck dissection.10 These recommendations were updated in 2002, with minor revisions of some boundaries by using radiologic landmarks and a better definition of sublevels of levels II and V (Table 4-1).11 More recently, the AHNS updated the guidelines on the classification and terminology of neck dissection (Table 4-2).12

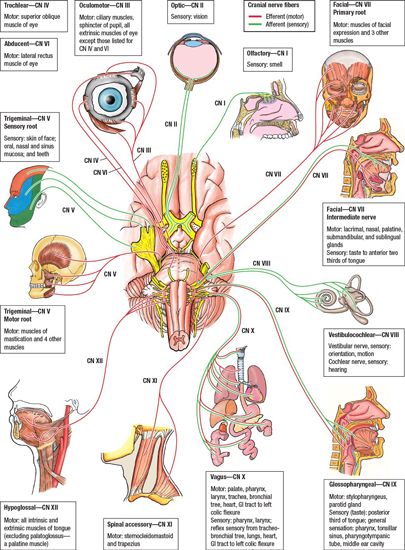
• The boundaries of regional nodal levels have been described by the American Joint Committee on Cancer (see Edge et al. 2010).13 The cervical nodes are shown in Figure 4-1. Figure 4-2 presents a graphical summary of the cranial nerves affected by head and neck cancer.
2. DETERMINATION OF CLINICAL TARGET VOLUMES
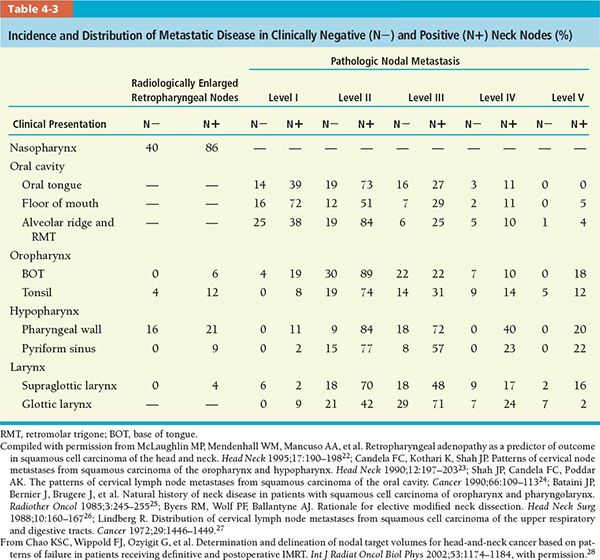
• Determination of CTVs is based on the data on incidence and location of metastatic neck nodes from various head and neck subsites, which come from the published literature and summarized in Table 4-3. The distribution of nodal metastasis to different lymph node levels in the head and neck region varies by primary tumor subsite. The number under each column represents the percentage of lymph node metastasis in patients with squamous cell carcinoma arising from various head and neck subsites. Because metastatic nodes may be manifest in more than one nodal level at presentation, the summation of percentage from all nodal levels (regions) may exceed 100%, especially in the N+ group.
• Other factors that influence the extent of CTV margins include tumor size, stage, differentiation, morphology, and whether other lymph node levels are involved.14,15
• Avoiding elective contralateral neck radiation is an area of great interest primarily to reduce significant morbidity without compromising clinical outcome. Earlier studies demonstrated that node-negative, well-lateralized tonsillar cancers had low contralateral neck failures.16,17 More recent prospective studies have shown that ipsilateral neck radiotherapy may have a role in the treatment of well-selected oral cavity and node-positive oropharyngeal cancer patients. Rusthoven et al. demonstrated that primary and ipsilateral neck radiation in node-positive, lateralized tonsillar cancer did not appear to increase the risk of contralateral nodal failure, although follow-up was short.18 Cerezo et al. showed that ipsilateral irradiation for well-lateralized oropharynx and oral cavity cancers spared salivary gland function without compromising locoregional control.19 While ipsilateral radiation is appropriate for selected cases, contralateral nodal regions should be included for tumors that tend to spread to the contralateral neck nodes or those that arise from or invade a midline structure, including the soft palate, base of tongue (BOT), posterior pharyngeal wall, or nasopharynx. For example, nodal metastasis exists in 85% to 90% of patients with nasopharyngeal carcinoma, and approximately 50% of them have bilateral disease; therefore, both sides of the neck should be treated for this particular disease. In addition, the risk of contralateral neck involvement is high in oral cavity cancers if tumor is above T3 classification, crosses the midline, or presents with ipsilateral node metastasis, which requires bilateral treatment of lymph nodes.20
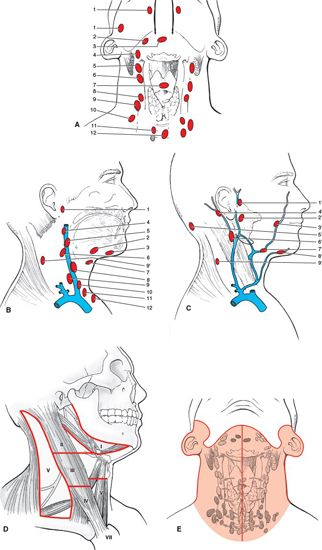
FIGURE 4-1. Nodal anatomy. The neck or cervical nodes are regional nodes for the head and neck cancer sites. Any cluster can be a sentinel node that drains a specific site (A, B, C). These are assigned using the International Anatomical Terminology and are identified by numbers to denote the clusters of regional nodes that could be sentinel nodes. (A) Anterior view of deep jugular nodes. (B) Lateral view of deep nodes with the sternocleidomastoid muscle removed. Anterior deep jugular (A) and lateral (no sternocleidomastoid muscle) (B) lymph nodes: (1) retropharyngeal; (2) submandibular; (3) submental; (4) superior deep cervical; (5) jugulodigastric; (6) mid deep jugular; (7) prelaryngeal; (8) jugulo-omohyoid; (9) inferior deep cervical (jugular); (10) supraclavicular; (11) paratracheal; and (12) pretracheal. C: Lateral view of superficial nodes: (1’) preauricular; (2’) parotid; (3’) facial; (4’) mastoid; (5’) superficial cervical; (6’) external jugular; (7’) submandibular; (8’) submental; and (9’) spinal accessory. (D) AJCC nomenclature of seven levels of cervical lymph nodes. (E) Node-bearing region according to staging in Hodgkin’s lymphoma. (Taken from Rubin P, Hansen JT. TNM Staging Atlas with Oncoanatomy, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:11.)
FIGURE 4-2. Graphical representation of the cranial nerves. (Modified from Agur AMR, Dalley, AF, eds. Grant’s Atlas of Anatomy, 12th edition. Philadelphia; Lippincott Williams & Wilkins, 2009.)
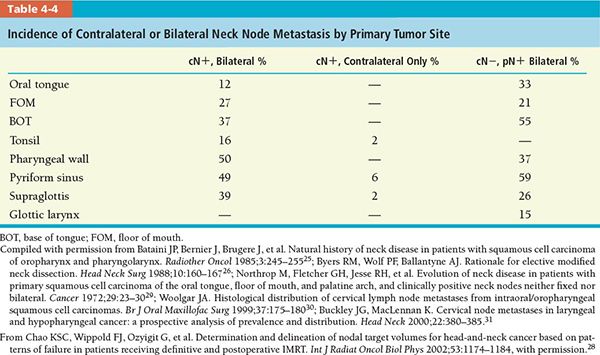
• Table 4-4 shows that macroscopic or microscopic bilateral nodal metastases present with more than 30% of tumor residing in the BOT, pharyngeal wall, and pyriform sinus.
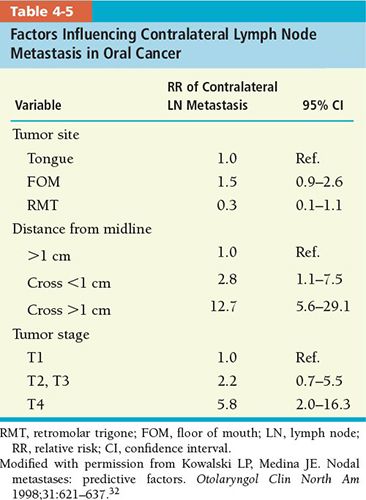
• The probability of contralateral nodal metastasis can be predicted with better accuracy if certain tumor characteristics are taken into account. Table 4-5 shows the tumor factors for oral cavity carcinoma that could influence the incidence of contralateral nodal metastasis.
• If a tumor arises from the buccal mucosa and retromolar trigone, which have a lower chance of contralateral neck node metastasis, especially when the primary tumor size is small and no involvement of the ipsilateral neck node is evident, the contralateral neck may not require treatment.
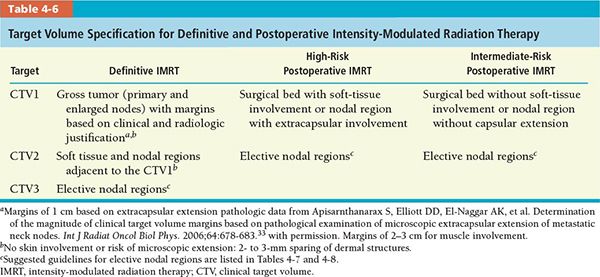
• We define three CTVs for target volume specification: CTV1, CTV2, and CTV3 (Table 4-6).
º CTVl for patients receiving definitive IMRT is defined as gross tumor volume (GTV) or nodal GTV with margins based on clinical and radiologic factors.
º CTVl for postoperative patients encompasses the preoperative GTV plus a 0.5 to 1.5 cm margin including the resection bed with soft tissue invasion by the tumor or extracapsular extension (ECE) by metastatic neck nodes. Preoperative imaging, surgical defects, or postsurgical changes seen on postoperative CT scan determine the surgical bed.
º CTV2 for patients receiving definitive IMRT encompasses the CTVl and the region adjacent to CTVl but with no direct tumor involvement based on clinical findings and CT or MRI imaging. Radiologically or clinically involved neck node is also included in CTV2 with 1-cm margins truncating air and bone.
º CTV2 for postoperative patients primarily includes the clinically/radiologically or pathologically uninvolved cervical lymph nodes, deemed as elective nodal regions, or the prophylactically treated neck.
º CTV3 for patients receiving definitive IMRT includes the clinically/radiologically or pathologically uninvolved cervical lymph nodes, deemed as elective nodal regions, or the prophylactically treated neck.
• Our approach to selective neck treatment was similar to that recommended by Gregoire et al.21 These target volume specifications were integrated with the published clinical data shown in Table 4-3. On the basis of these historical data, we proposed that treatment of the N0 neck is warranted if the probability of occult cervical metastasis is higher than 5% to 10%.
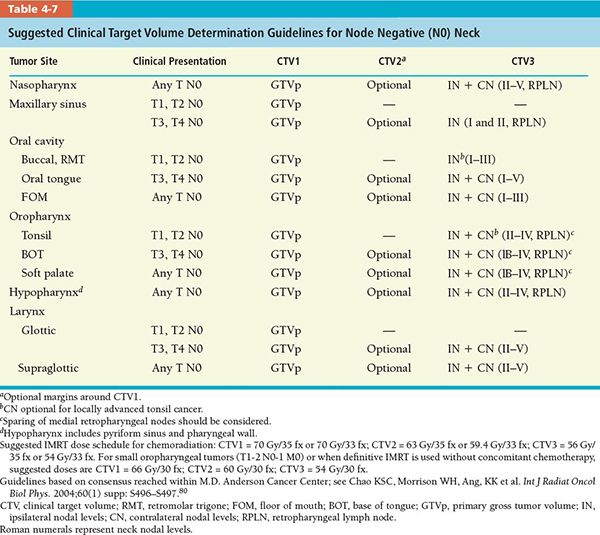
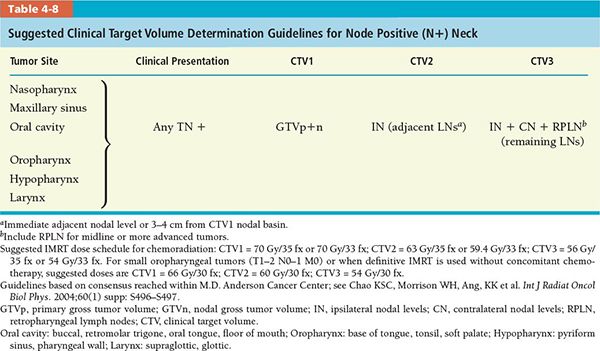
• The CTV (CTV1, CTV2, and CTV3) determination guidelines for various head and neck tumor subsites for postoperative and definitive IMRT are divided into separate recommendations for node negative and node positive neck, which are summarized in Tables 4-7 and 4-8, respectively.
• IMRT is applied to the upper neck for salivary sparing. The lower neck is treated with a conventional AP lower neck port if indicated. The standard superior border for the lower neck field is at the level of the thyroid notch. In patients with a tumor or metastatic lymph node extending below this level, the junction line is adjusted to avoid bisecting gross disease.
3. DELINEATION OF CLINICAL TARGET VOLUME
• Because the definition of neck node levels and the anatomic boundaries described in Robbins’ classification were designed on the basis of specific soft-tissue landmarks for surgical procedures and are not easily seen on CT and MRI slices, we implemented modified guidelines for the delineation of the various node levels in the neck. We supplemented Robbins’ classification with a retropharyngeal nodal group to provide the readers a better understanding of the nodal target volume determination and delineation for head and neck IMRT. Our recommendations for the radiologic boundaries of these nodal levels are summarized in Table 4-9.
º Level la: Submental; see figure 3 of Reference 15.
• Contains the submental nodes.
• Drains the skin of chin, mid-lower lip, tip of the tongue, and anterior floor of the mouth.
• Greatest risk of harboring metastases from the lower lip, floor of the mouth, anterior oral tongue, and anterior alveolar mandibular ridge.
º Level lb: Submandibular; see figure 3 of Reference 15.
• Contains the submandibular nodes.
• Drains the medial canthus, lower nasal cavity, hard and soft palate, maxillary and alveolar ridges, cheek, upper and lower lips, and most of the anterior tongue. It also receives efferent lymphatics from the submental nodes.
• Greatest risk of harboring metastases from the oral cavity, anterior nasal cavity, soft tissue structures of the mid-face, and submandibular gland.
º Level IIa: Ventral upper jugular group; see figures 2 through 6 in Reference 15.
• Contains upper one-third of jugular lymph nodes anterior to the jugular vein.
• Receives efferent lymphatics from the face; parotid gland; and submandibular, submental, and retropharyngeal lymph nodes. It also directly drains from the nasal cavity, pharynx, larynx, external auditory canal, middle ear, and sublingual and submandibular glands.
• Greatest risk of harboring metastases from the oral cavity, nasal cavity, nasopharynx, oropharynx, hypopharynx, larynx, and major salivary glands.
º Level IIb: Dorsal upper jugular group; see figure 4 of Reference 15.
• Contains upper one-third of jugular lymph nodes posterior to the jugular vein.
• Greatest risk of harboring metastases from the oropharynx and nasopharynx, and less likely from the oral cavity, larynx, and hypopharynx.
º Level III: Middle jugular group; see figures 3, 4, 6, and 7 of Reference 15.
• Contains middle jugular lymph nodes.
• Drains from the BOT, tonsils, larynx, hypopharynx, and thyroid gland. Receives efferent lymphatics from levels II and V, and some from the retropharyngeal, pretracheal, and recurrent laryngeal nodes.
• Greatest risk of harboring metastases from the oropharynx, nasopharynx, oral cavity, larynx, and hypopharynx.
º Level IV: Inferior jugular group; see figure 6 of Reference 15.
• Contains the inferior jugular lymph nodes.
• Receives efferent lymphatics from levels III and V, and some from the retropharyngeal, pretracheal, and recurrent laryngeal nodes.
• Greatest risk of harboring metastases from the larynx, hypopharynx, and cervical esophagus.
º Level V: Posterior triangle group; see figure 8 of Reference 15.
• Contains the posterior cervical (Level Va) and supraclavicular lymph nodes (Level Vb).
• Drains from the occipital scalp, postauricular and nuchal regions, skin of the lateral and posterior neck, nasopharynx, and oropharynx (tonsil and BOT).
• Greatest risk of harboring metastases from the nasopharynx, oropharynx, subglottic larynx, apex of pyriform sinus, cervical esophagus, and thyroid gland.
º Level VI: Anterior compartment; see figure 8 of Reference 15.
• Contains the lymph nodes of the anterior compartment from the hyoid bone superiorly to the suprasternal notch inferiorly. On each side, the lateral border is formed by the medial border of the carotid sheath.
• These include LN cervicales anteriores superficiales and LN cervicales anteriores profundi (LN infrahyoidales, LN prelaryngeales, LN pretracheales, LN paratracheales, and LN thyroidei).
º Retropharyngeal nodes:
• Contains the lymph nodes that lie within the retropharyngeal space.
• Retropharyngeal nodes are divided into lateral and medial groups. There is data to suggest that sparing medial retropharyngeal nodes is safe in node-negative oropharyngeal cancers.34
• Greatest risk of harboring metastases from the nasopharynx, oropharynx, soft palate, hypopharynx, and pharyngeal tumors with positive neck nodes in other levels.
• In addition to the LN regions classified by Robbins et al., there are other LN regions which include the parotid level (LN parotidei superficiales and profundi, which drain to level IIa), the retroauricular level (LN retroauriculares), the retropharyngeal level (LN retropharyngeales), the buccales level (LN facials, which drains to level Ib), and the external jugular level (LN cervicales laterals superficiales, which drain to level III). See figure 9 of Reference 15.
• Two summary figures from Reference 15 are reprinted here as Figures 4-3 and 4-4.
• In view of the differences observed between recent guidelines, a multidisciplinary working party including members of both groups was created to try to elaborate a unique set of recommendations for the delineation of the various levels in the node negative neck. Subsequently, the party was extended to include representatives of American and European cooperative groups.
• The general objectives that guided the activities of the panel were to translate as accurately as possible the surgical guidelines into a set of radiologic guidelines by using axial CT sections and to minimize differences in interpretation of the guidelines by defining less ambiguous boundaries than previously described.
• As a result of this consensus meeting, a complete atlas of contrast-enhanced CT sections depicting the various node levels from the base of the skull to the level of the sternoclavicular joint for the node negative neck has been created, which is posted on the website of the Danish Head and Neck Cancer Study Group, figure 4-3A,B (http://www.dshho.suite.dk/dahanca/guidelines.html).
• Figure 4-5 gives an atlas of CTV delineation for head and neck squamous cell carcinoma. Figure 4-6 illustrates an example of sequential steps in delineating the CTVs.
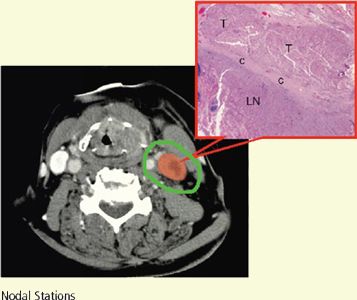
• One significant pathologic factor to take into consideration for target volume delineation is the presence of ECE. The probability of the tumor extending outside of the nodal capsule increases as a function of tumor size. When metastatic nodal disease expands and ruptures the capsule of cervical lymph nodes, the incidence of local recurrence increases.

• Huang et al.35 demonstrated a higher tumor recurrence in patients with ECE (+) neck, whereas postoperative radiation therapy improved local regional control. Peters et al.36 differentiated the resected neck into high- and low-risk groups, for which different radiation doses were recommended.
• Thus, when delineating target volume in the postoperative neck, inclusion of generous soft-tissue margins around the tumor bed is imperative. If the information regarding which lymph node levels contain ECE (+) nodes is not available pathologically, a preoperative imaging study (CT or MRI) can assist in determining which regions require a more generous soft-tissue margin for CTV1 and CTV2 delineation.
• Figure 4-7 differentiates ECE (+) and ECE (−) necks in patients receiving postoperative IMRT. In the ECE (+) neck, soft tissues are more generously included, and the CTV1 and CTV2 should extend close to the skin surface, especially in the region or level of the ECE (−) node(s) specified by the pathologic findings.
• When postoperative IMRT is required for the ECE (−) neck, the target volume should avoid skin surface to decrease acute dermal toxicity. In our experience, sparing 2 to 3 mm of dermal structures in target volume design (Fig. 4-7) clearly results in much better radiation tolerance, less treatment breaks, and no compromise in locoregional control.
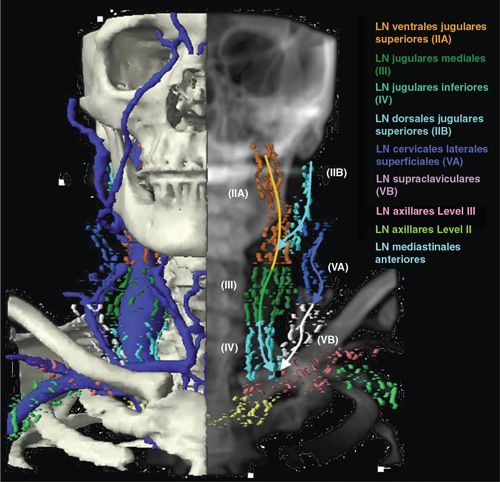
FIGURE 4-3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree