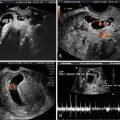
Fig. 7.1
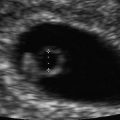
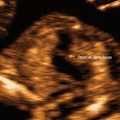
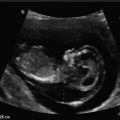
Normal early gestational sac. (a) Transabdominal transverse image at 5 weeks, 1 day, showing an apparently empty early gestational sac (white arrow) eccentrically located within the decidua (black arrow). Transvaginal transverse (b) and sagittal (c) images at 5 weeks, 1 day can show the yolk sac within the early gestational sac, thus confirming an early IUP. (d) Transvaginal transverse image at 5 weeks, 1 day shows an eccentric round fluid collection with an echogenic rim (white arrowhead) adjacent to the echogenic line representing the collapsed endometrial cavity (black arrowhead) demonstrating the “intradecidual sign.” (e) Transvaginal transverse image at 6 weeks, 5 days, showing an intrauterine fluid collection surrounded by two concentric echogenic rings forming the so-called “double-sac sign”
An important distinction in early pregnancy ultrasound is the ability to differentiate a true intrauterine gestational sac, before the yolk sac or embryo is seen, from an intrauterine fluid collection such as a subendometrial cyst, decidual cyst or fluid collection in the setting of an ectopic pregnancy. Careful interrogation will demonstrate that subendometrial cysts are external to the decidualized endometrium (Fig. 7.2). A decidual cyst is also a benign finding but is more challenging to distinguish from an early IUP. The key distinction will depend on the relationship of the early IUP, which should abut the interstitial line of the collapsed endometrial cavity (see Fig. 7.1d), whereas the decidual cyst may not be anatomically related to the collapsed endometrial cavity. In cases where there is doubt, follow-up imaging will demonstrate appropriate growth of the early IUP gestational sac, with development of a yolk sac to confirm an early IUP.
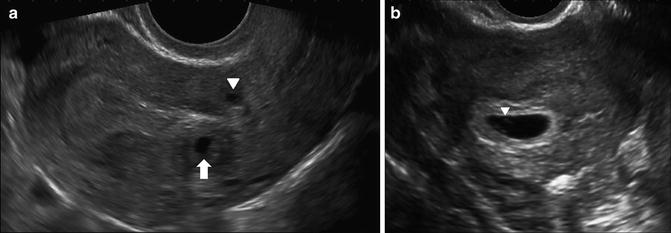

Fig. 7.2
Subendometrial cysts in a patient with a positive pregnancy test and unknown dates, transvaginal images. (a) Sagittal image shows a rounded fluid collection posterior to the hyperechoic decidualized endometrium (arrow) and a similar cyst anterior to the endometrium in the lower uterine segment (arrowhead). These are incidental findings. No gestational sac was seen at this time. (b) Follow-up study 9 days later shows intrauterine gestational sac with visible yolk sac (arrowhead)
It is equally important to ensure the “cyst” is not actually within the endometrial cavity. In most cases, fluid within the endometrial cavity can be distinguished from fluid outside of the endometrial cavity on the basis of shape, location and contents. Whereas endometrial cavity fluid collections may be angular, complex with internal echogenic debris, or elongated, conforming to the uterine cavity, the early gestational sac will be anechoic and typically rounded or ovoid [10]. In cases where there is uncertainty, the differential diagnosis will include a pregnancy of unknown location or intracavitary fluid collection, accompanying early pregnancy loss.
Recent studies have shown that intrauterine fluid, commonly referred to as a “pseudogestational sac,” in the setting of ectopic pregnancy occurs much less frequently than previously thought and may be seen in only around 10 % of ectopic pregnancies [11, 12]. Doubilet et al. reported in an editorial on pregnancy of unknown location that given the relatively low incidence of ectopic pregnancy (approximately 2 %) and low likelihood of intrauterine fluid with an ectopic pregnancy, the probability that any intrauterine fluid collection in a woman with a positive pregnancy test represents a gestational sac is 99.5 % [13]. This has led to a change in thinking with respect to early pregnancy such that, in the absence of sonographic evidence of ectopic pregnancy, any fluid collection with curved edges in a woman with a positive pregnancy test should be thought of as an early intrauterine gestational sac [13]. In this setting, follow-up imaging can be obtained, to confirm subsequent appearance of embryonic structures including a yolk sac and an embryo with cardiac activity. The role of follow-up β-hCG serology is this setting is discussed separately.
The double sac sign (DSS) and intradecidual sign (IDS) have historically been described as useful in differentiating a true IUP from a non-gestational intrauterine fluid collection [14, 15]. The DDS, first described in 1982, refers to the appearance of two echogenic rings around the gestational sac felt to represent the inner and outer layers of the decidua, the decidua capsularis and decidua basalis (see Fig. 7.1e) [15]. The IDS, initially described in 1986, refers to a fluid collection with an echogenic rim in an eccentric location on one side of the uterine cavity (see Fig. 7.1d) [14]. Both of these signs were initially described on transabdominal ultrasound and considered to be early reliable signs of an IUP with the DSS seen in 77 % of the initial study population with an IUP and the IDS seen in 92 % [13–15]. Currently, with the advent of high-resolution, high-frequency transvaginal transducers, subsequent studies have shown that in normal IUP the DSS may be absent in 50–60 % and the IDS absent in up to 50 % [13, 16, 17]. With improvements in technology, it is no longer infrequent that the yolk sac is visible prior to DSS and IDS and, consequently, these signs are now felt to be of limited utility. While their presence may be useful in confirming an IUP, the absence of a DSS or IDS should not be used to exclude one [18].
Measurement of the Gestational Sac1
Measurements of the gestational sac can be obtained and used to estimate gestational age and predict appearance of normal embryonic structures. The mean sac diameter (MSD) is obtained by averaging the transverse, sagittal and anteroposterior dimensions of the gestational sac and can be correlated with expected GA and with β-hCG levels (Fig. 7.3). There is variability in gestational sac size measurements between different observers. This was highlighted in a recent study of 54 patients by Pexsters et al. [19] which reported the interobserver variability for MSD as being up to ±19 %. Expected rate of growth of the gestational sac has previously been reported as approximately 1 mm per day [7]. However, in a study of 359 women, Abdallah et al. [20] found overlap in MSD growth rate in viable and nonviable early IUP and were unable to define a rate of gestational sac size that can be considered normal in early pregnancy [20]. A small gestational sac with less than 5-mm difference between crown-rump length and gestational sac length has been referred to as first-trimester oligohydramnios and considered to be associated with poor outcome [21]. However, this has only been examined in a small population and as an isolated finding likely warrants follow-up imaging (Fig. 7.4).
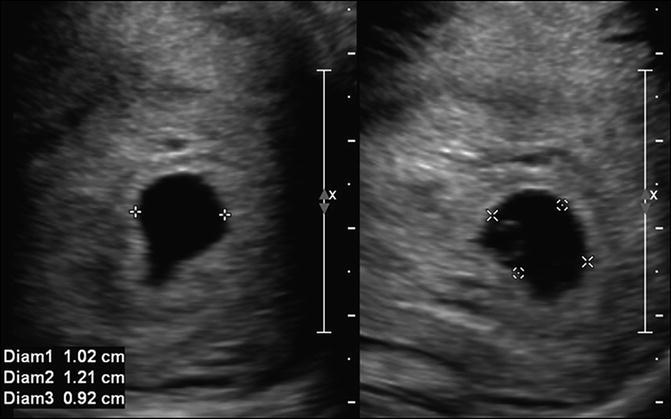
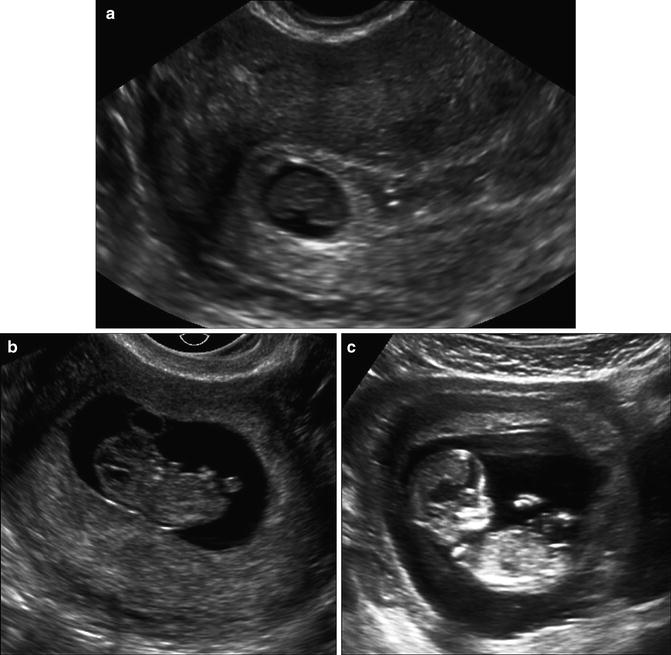

Fig. 7.3
Mean sac diameter. Transabdominal sagittal and transverse images at 5 weeks, 5 days of the uterus, measuring the gestational sac in three orthogonal dimensions, which are averaged to obtain the mean sac diameter. Care should be taken to place calipers directly on the white line around the sac to ensure accurate measurements

Fig. 7.4
First-trimester oligohydramnios. (a) Transvaginal image at 7 weeks, 4 days, showing small sac size (MSD 12.4 mm) for CRL (8.1 mm) in keeping with “first-trimester oligohydramnios.” (b) Transvaginal follow-up scan at 8 weeks, 1 day shows live embryo with persistent discordant GS size. (c) Transabdominal image at 12 weeks, 0 days shows live fetus with interval growth of both sac and fetus; however, sac continues to be small. A follow-up study at 16 weeks demonstrated normal fetal growth and fluid
Mean Sac Diameter and Viability
Much attention has been given to the maximum size at which an empty gestational sac, i.e., a sac without a visible yolk sac or embryo, can be considered normal. While a yolk sac is usually seen with an MSD > 8 mm, the MSD at which a yolk sac and embryo can be sonographically detected is variable and caution should be exercised if using MSD to determine viability [22]. Historically, the upper limit at which an empty gestational sac was considered a normal early pregnancy finding was a transvaginally measured MSD between 16 and 20 mm [23, 24]. However, several recent studies have shown that a small percentage of viable pregnancies may exist with empty sac size up to 18–19 mm [25, 26] and given interobserver variability in sac size measurement, an empty gestational sac should be considered a potentially normal early pregnancy finding up to an MSD of 25 mm on transvaginal ultrasound [20, 23, 24, 26, 27]. This is discussed further in Chap. 10 which refers to new discriminatory criteria for defining early viability. Detection of peritrophoblastic flow demonstrating high velocity and low impedance around the gestational sac is a normal finding that has been described as an aid in confirming a very early intrauterine pregnancy [28]. However, caution is recommended in the use of color and, particularly, spectral Doppler imaging in early pregnancy, due to increased power output and potential risk to developing pregnancy [8, 29].
Gestational Sac Appearance and β-hCG in Early Pregnancy
A gestational sac is usually visible in an intrauterine pregnancy when the β-hCG level is between 1000 and 2000 mIU/ml [4]. Historically, much attention has been paid to the concept of a discriminatory β-hCG level above which an intrauterine gestational sac should always be seen in a normal IUP. This number was initially around 2000 mIU/ml, however, it is now recognized that there is considerable overlap of β-hCG levels in viable IUP, nonviable IUP and ectopic pregnancy [30, 31]. Doubilet et al. have reported that, in rare instances, even with an absent gestational sac on transvaginal ultrasound and β-hCG level >4000 mIU/ml, follow-up ultrasound can show a normal pregnancy [30]. Practically speaking, it is unlikely to have a normal IUP development when no gestational sac is seen with a β-hCG level >3000 mIU/ml. This cannot, however, be considered diagnostic criteria for early pregnancy loss with 100 % specificity. Consequently, the use of a single discriminatory β-hCG level to guide management in the setting of pregnancy of unknown location (PUL) is not recommended [30, 32]. There is emerging evidence that the incremental increase in serum β-hCG over 48 h, expressed as a ratio, may be a useful predictor of IUP when ultrasound findings are inconclusive [33–35]. Recently, Bignardi et al. [33] have reported that a β-hCG ratio >2.0 is suggestive of a viable IUP with sensitivity of 77 %, specificity of 96 % and a positive predictive value of 87 %. However, the use of β-hCG ratio as a predictor of viability in the setting of early pregnancy is not yet routine practice in all centers.
Yolk Sac
The appearance of the yolk sac provides the first definitive confirmation of an intrauterine pregnancy. Although visualization of the gestational sac, the double decidual sac sign, or the intradecidual sign may increase the likelihood of an IUP, they are not as accurate at confirming an IUP as detection of the yolk sac.
The yolk sac can first be visualized within the gestational sac at approximately 5.5 weeks gestation [1, 5, 8, 36] as a thin echogenic circular ring within the gestational sac. It is usually visible by the time the MSD reaches 8 mm. If the yolk sac is not identified by this stage, a careful interrogation of the gestational sac with image optimization may assist in the ability to detect it. These may include narrowing the sector width, image zoom, appropriate placement of the focal zone, choice of a higher frequency, or other vendor specific post-processing settings (Fig. 7.5).
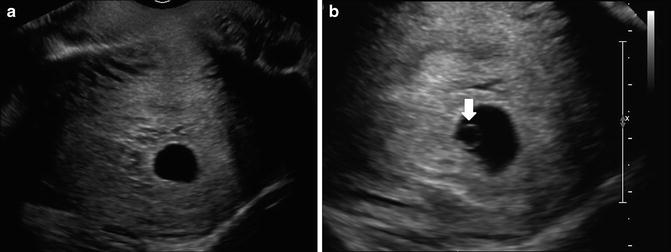

Fig. 7.5
Normal yolk sac at 6 weeks, 1 day. (a) Trans-vaginal transverse image shows an apparently empty gestational sac. (b) Transvaginal transverse zoomed image with narrowed sector width is able to detect a circular echogenic ring, which represents the yolk sac (white arrow) and thus confirms an early IUP
Normal yolk sac measurement between 5 and 10 weeks GA is around 5 mm [8]. An enlarged yolk sac greater than 5 mm may be associated with poor pregnancy outcome. As an isolated finding, however, it cannot be considered definitely abnormal (Fig. 7.6) [37].
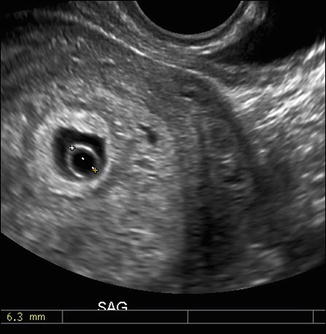

Fig. 7.6
Transvaginal sagittal image of large yolk sac at 5 weeks, 6 days shows enlarged yolk sac measuring 6 mm
Embryo
The embryo is first visualized alongside the yolk sac at approximately 6 weeks gestation. Initially, it appears as a featureless linear echogenic structure without discernible limb buds and with no distinct cranial or caudal end (Fig. 7.7a). At this stage a quantitative assessment of the embryo is achieved by obtaining the longest length measurement. Once the crown and rump are distinguishable, it is ideal to acquire the crown-rump length (CRL), which is defined as the longest length excluding the limbs and yolk sac (Fig. 7.7b). The CRL can be measured transabdominal or transvaginal. The ideal plane of measurement is midsagittal, with the embryo or fetus in a neutral position. Presence of fluid between the fetal chin and chest can be used as a sign to ensure that the fetus is not hyperflexed [2]. In practical terms, prior to 7 weeks gestation, measurement is actually of the longest length of the embryo that can be seen, but, after 7 weeks, a concerted effort should be made to measure the embryo in the midsagittal plane, excluding the yolk sac [38] (Fig. 7.7c). The smallest detectable embryo transvaginally has a CRL of 1–2 mm. Normograms are available to correlate CRL with gestational age [39–42]. A recent 2014 publication by Papageorghiou et al. provides a CRL chart for pregnancy dating, based on a multicenter international trial, thus providing a first international standard for evaluating CRL linear growth in first trimester [43].
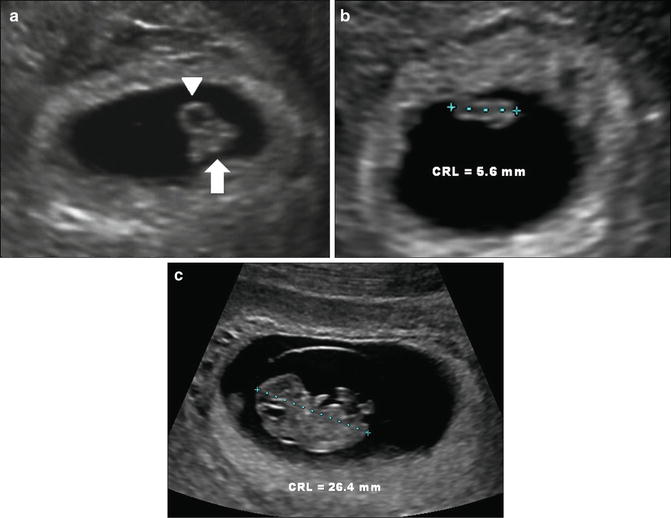

Fig. 7.7
Early embryo and crown-rump length. (a) Transvaginal image at 6 weeks, 6 days, showing yolk sac (arrowhead) and embryo (arrow). (b) Embryo at 6 weeks, 2 days with CRL of 5.6 mm. The maximum linear length is measured. (c) Embryo at 9 weeks, 2 days with CRL of 26.4 mm. Note that distinct cranial and caudal ends are now visible. Calipers must be carefully placed to exclude yolk sac and flexed limbs
Embryonic and Fetal Cardiac Activity
Embryonic cardiac activity (ECA) is usually visible as soon as the embryo is detectable and can be seen with a CRL as small as 1 mm [4]. Although, ECA is virtually universally identified by CRL of 4–5 mm, the absence of ECA cannot be considered abnormal until the embryo reaches 7 mm [23, 24]. This number is chosen to achieve as close to 100 % specificity as possible for diagnosing early pregnancy loss based on absence of embryonic cardiac activity while taking into account the potential ±15 % interobserver variability in CRL measurement [44].
Embryonic cardiac activity is documented using motion mode (M mode) to determine a heart rate (Fig. 7.8). ECA is considered normal if greater than 100 beats per minute (bpm). Doubilet et al. [45] suggested a lower limit of normal embryonic heart rate of 100 bpm up to 6.2 weeks gestation and 120 bpm between 6.3 and 7 weeks. Embryonic heart rates <100 bpm were thought to be associated with an increased risk of demise. However, a recent review of early first-trimester pregnancies with slow embryonic heart rate reveals that this may not necessarily be a poor prognostic factor [45–47]. High embryonic heart rate in early pregnancy has been defined by Benson et al. [48] as >135 bpm before 6.3 weeks and >155 bpm between 6.3 and 7 weeks. This generally has a good prognosis with high likelihood of normal outcome.


Fig. 7.8
M mode of embryonic cardiac activity at 6 weeks, 6 days. Transvaginal image showing embryonic cardiac activity detected with M mode
Multiple Gestations and Chorionicity2
Globally twins are approximately 1–3 % of all pregnancies [49]. Twin rates have doubled between 1980 and 2009 from 18.9 to 33.2 per 1000 births [50]. In some areas of the USA the prevalence of multiple gestations is as high as 1 in 30 pregnancies. This is thought to be related to a combination of assisted reproductive technologies and increasing maternal age. Two-thirds of twin pregnancies are dizygotic and one-third is monozygotic. The rate of intrapartum complications including pregnancy loss due to twin-twin transfusion syndrome or selective fetal growth restriction is far greater in monochorionic twins [51]. For optimal care, monochorionic pregnancies should be identified as early as possible to allow for closer surveillance and early detection of these conditions. Assignment of chorionicity is therefore a mandatory and vital component of first-trimester ultrasound with multiple gestations. However, surprisingly in a review by Wan et al., only 44 % of pregnancies referred to a tertiary care center had accurate diagnosis of amnionicity and chorionicity [52]. It is therefore important that practitioners be familiar with signs used to assess amnionicity and chorionicity in the first trimester.
The best time to determine chorionicity is prior to 14 weeks gestational age [51]. The classic features to determine a dichorionic gestation include two separate gestational sacs or placental masses, a thick inter-twin membrane in association with the lambda (λ) sign and different fetal genders. The λ sign refers to a triangular shaped projection of tissue which extends into the inter-twin membrane and is synonymous with the “twin-peak” sign (Fig. 7.9).


Fig. 7.9
Lambda sign in 11 weeks, 3 days dichorionic twin gestation. (a) Transabdominal image showing triangular shaped tissue projecting into inter-twin membrane (white outline) representing the lambda sign. (b) 3D representation showing lambda sign (arrow) and thick inter-twin membrane (arrowhead)
Prior to 10 weeks the presence of two distinct, separated gestational sacs will confirm a dichorionic–diamniotic (DCDA) pregnancy. After 10 weeks, the most reliable signs for assessing chorionicity are a combination of placental number and the λ sign [51] given that gender cannot be reliably be determined prior to 12 weeks. In a study of 648 twin pregnancies, the number of placental masses and the presence of either the T or λ sign is virtually 100 % accurate for determining chorionicity between 11 and 14 weeks [51].
With respect to differentiating monoamniotic from diamniotic pregnancies, it is important to recognize that the temporal development of the yolk sac and the amnion is variable [53]. The appearance of the amniotic sac can be as late as 8–10 weeks with the thin membranes making it challenging to identify, even with the higher resolution of transvaginal ultrasound (Fig. 7.10). In the setting of multifetal pregnancies, the number of yolk sacs was previously thought to be a reliable way to assign amnionicity; however, it is less reliable than once presumed [53, 54]. While the presence of two yolk sacs is highly predictive of a diamniotic pregnancy and is seen in approximately 85 % of monochorionic–diamniotic (MCDA) twins, the presence of only one yolk sac can be seen with both monoamniotic and diamniotic twin pregnancies [53]. On rare occasion a monochorionic–monoamniotic (MCMA) twin pregnancy may present with two yolk sacs. If it is uncertain that a membrane is present separating the fetuses, it is prudent to repeat the test 1–2 weeks later. Alternatively, direct visualization of umbilical cord entanglement may provide early confirmation of the monoamniotic status of a twin gestation.


Fig. 7.10
Early MCDA twin pregnancy. (a) Transvaginal transverse image at 7 weeks, 0 days shows two yolk sacs (arrows) in keeping with twin gestation. No dividing membrane is seen; however, amnionicity cannot be definitely assigned at this early stage. (b) Follow-up study at 8 weeks showing two yolk sacs and two embryos. A dividing membrane is not yet identified. (c) Thirteen-week follow-up shows thin dividing inter-twin membrane confirming MCDA pregnancy
Heterotopic Pregnancy
Prior to the more widespread use of assisted reproductive techniques (ART), the presence of an intrauterine gestational sac with a yolk sac was felt to virtually exclude the diagnosis of ectopic pregnancy. Nonetheless, heterotopic pregnancy can occur where an ectopic pregnancy coincides with an otherwise normal IUP. The estimated incidence of this occurrence is between 1 in 8000 and 1 in 30,000 (Fig. 7.11) [55]. The incidence may be higher in gestations after assisted fertility [56] and has been estimated as high as 1 in 100 in this population [40]. Thus, in patients who have undergone ART, despite the presence of an IUP, a thorough interrogation of the adnexae is recommended, to rule out a heterotopic pregnancy.
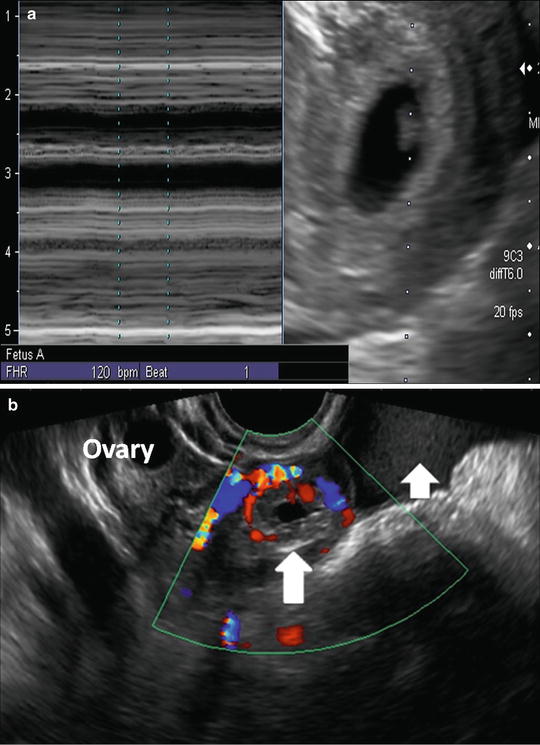

Fig. 7.11
Heterotopic pregnancy. (a) Transvaginal image at 6 weeks, 5 days showing live intrauterine pregnancy. (b) Transvaginal sagittal image of the right adnexa shows a hypoechoic mass (long arrow) surrounded by a vascular echogenic ring, i.e., “ring of fire,” separate from the normal ovary with follicles visible at superior margin image. Complex free fluid in keeping with hemorrhagic fluid (short arrow) is noted
Early Pregnancy Dating
One of the indications of first-trimester ultrasound is to confirm dating of pregnancy. Accurate pregnancy dating is critical to prenatal management for a variety of reasons including: to prevent preterm induction of supposed postdates pregnancies, to determine viability in the setting of premature delivery, to interpret growth patterns, to optimize prenatal screening for aneuploidy and to appropriately time diagnostic interventions such as chorionic villus sampling and amniocentesis [57, 58]. Evidence has shown early ultrasound dating of pregnancy to be more accurate at predicting the expected due date than menstrual history [2, 59, 60]. Pregnancy dating is most accurate in the first trimester and can be determined using MSD, CRL, or fetal biometry. MSD can be used for dating when the embryo is not seen but shows greater variability in predicting GA compared to CRL [61] and should not be used when an embryo is visible. The most accurate estimation of gestational age is achieved in the first-trimester by using the CRL somewhere between 8 weeks and 13 + 6 weeks [2, 42, 58, 62]. At earlier gestations, the relatively small size of the embryo may lead to more significant measurement error. The reported accuracy of the CRL measurement for dating is within 3–8 days [7], with the most accurate results reported when CRL measures [63] between 7 and 60 mm [41, 42, 62]. CRL continues to be the most reliable predictor of gestational age until 12–14 weeks, when CRL and biometry begin to achieve similar accuracy [64–67]. Based on current recommendations by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), CRL is recommended for dating until the embryo measures 84 mm. Beyond 84 mm, the use of head circumference has been shown to be slightly more accurate than BPD [2]. Given the increased accuracy of ultrasound for pregnancy dating and the clinical importance of accurate dating, it has been suggested that a first-trimester dating ultrasound be performed in all pregnancies [63, 68–71]. Dating of the pregnancy may be performed concurrently with the 11- to 14-week nuchal translucency scan.
Thresholds for Assessing Viability
Recently, new diagnostic criteria have been published with respect to assessing early viability which will be discussed further in Chap. 10. These values have been chosen to include almost all normal pregnancies in an effort to “do no harm” and prevent the small risk of erroneously reporting early pregnancy loss in the setting of a viable pregnancy.
The criteria, which are based on transvaginal ultrasound assessment, have increased the thresholds at which nonvisualization of ECA and embryonic structures may be considered normal and are summarized as follows:
Absence of cardiac activity in an embryo < 7 mm may be normal .
Absence of an embryo with an MSD < 25 mm may be normal .
Follow-up in 1 week’s time is recommended in the above two scenarios.
Nuchal Translucency Evaluation3
Assessment of nuchal translucency is routinely offered in many countries, including the USA, as a component of prenatal screening and when combined with maternal age and maternal serum biochemistry (β-hCG and pregnancy associated plasma protein-A [PAPP-A]) can be an effective method of screening for chromosomal abnormalities [72]. Nuchal translucency (NT) refers to the sonolucent area posterior to the fetal neck. For the purpose of prenatal screening, the NT should be assessed between 11 and 13 + 6 weeks gestational age, when the embryo measures between 45 and 84 mm by transabdominal ultrasound technique. The NT should be seen transabdominally in about 80 % of cases [72]. Transvaginal assessment of the NT can be attempted if visualization is inadequate by transabdominal approach, however, it is more challenging due to limited ability to maneuver the probe to obtain a true midline sagittal image. If the NT is not adequately seen transabdominally, our routine is to either bring the patient back later the same day or on a subsequent day, rather than perform a transvaginal study. However, many centers will prefer to proceed to a transvaginal examination immediately following an unsuccessful transabdominal NT evaluation.
Criteria for NT Measurement
Accurate measurement of nuchal translucency is required to optimize results of screening tests. Both the American Institute of Ultrasound in Medicine (AIUM) and ISUOG have published guidelines outlining proper technique for NT measurement [2, 5]. The NT should be evaluated in the midsagittal plane of the face, defined by visualization of the echogenic tip of the nose and rectangular shape of the palate [2]. The fetus should be in a neutral position, neither hyperflexed nor extended. The image should be magnified, such that the fetal head and upper thorax fill the screen. Margins of the NT edges must be clear enough for proper placement of calipers which should be placed directly on the edges of the NT. Equipment used should allow for precise measurement up to 0.1 mm. The amnion should be seen as a separate echogenic line from the NT (Fig. 7.12). The NT should be measured at the widest space and, if multiple measurements meeting the criteria are obtained, the largest measurement should be used for risk assessment. It is important that individuals who perform a NT evaluation have undergone training and are associated with an appropriate quality assurance program.


Fig. 7.12
Nuchal translucency at 12 weeks, 0 days. Transabdominal sagittal midline image showing normal nuchal translucency (between calipers). The thin echogenic line of the amnion (white arrow) can be seen as separate from the NT
Significance of Elevated NT Measurement
Normal nuchal translucency is defined as a measurement less than 3 mm, if the 95th percentile is used or 3.5 mm, if the 99th percentile is chosen [72]. Nuchal translucency increases with increasing gestational age and higher measurements are associated with greater risk of abnormality [73]. When combined with maternal age and serology (including PAPP-A and maternal serum β-hCG), NT thickness successfully identified 89 % of fetuses with trisomy 21, with a false-positive rate of 5 % [72]. Elevated nuchal translucency can also be associated with other chromosomal abnormalities, including trisomy 13, 18 and Turner’s syndrome [72]. Even in normal karyotype fetuses, an elevated NT confers a greater risk of fetal structural anomalies, most commonly congenital heart anomalies [74]. The prevalence of congenital heart disease with an NT > 95th percentile is 1/48 and is 1/19 with NT >99th percentile [74]. A meta-analysis of 2271 singleton euploid fetuses, with NT > 3 mm at 10–14 weeks, reported structural anomalies in 10.6 % and genetic syndromes and single-gene disorders in 15 % [75]. The prevalence of abnormal outcome was shown to increase with increasing NT measurements.
It is important to note, however, when counselling patients, that not all elevated NT pregnancies are necessarily associated with congenital or structural anomalies (Fig. 7.13). In one report, 90 % of pregnancies with NT measurement below 4.5 mm and normal karyotype resulted in healthy live births [72]. Normal outcome was seen in 80 % of pregnancies with NT between 4.5 and 6.4 mm and 45 % of pregnancies with NT greater than 6.5 mm [76]. In the previously described meta-analysis evaluating outcome of elevated NT above 3 mm, chromosomally normal fetuses had a 68 % overall chance of normal outcome. Long-term neurodevelopmental outcomes have also been evaluated in children who had an increased fetal NT and normal karyotype. In a systematic review of 17 studies and 2458 patients, there was no significant difference in the rate of neurodevelopmental delay in this group, when compared to the general population., Nonetheless, further large-scale prospective studies are needed to predict neurodevelopmental outcome in this population with greater certainty [77].


Fig. 7.13
Elevated nuchal translucency in euploid fetus: fetus at 11 weeks, 1 day with abnormal elevated NT measuring 3.9 mm (arrow). Note amnion is visualized separate and distinct from amnion (dashed arrow). Subsequent cell-free DNA, chorionic villus sampling, early anatomy including echocardiography were normal. The pregnancy continued with normal outcome
Recommendations in the setting of increased NT include detailed early anatomic assessment to look for structural anomalies, fetal echocardiography, and discussion regarding cell-free DNA analysis and/or invasive diagnostic testing such as chorionic villus sampling and amniocentesis.
Assessment of the Nasal Bone During the NT Evaluation
Evaluation for presence or absence of the nasal bone can be performed at the time of NT scan [72, 78, 79]. Nasal bone ossification first becomes apparent at a crown rump length of approximately 42 mm [79] or 11 weeks gestation and nasal bone length progressively increases with gestation. Assessment for presence of the nasal bone is performed in the midsagittal plane and, as for NT measurement, requires strict adherence to proper technique and operator experience to be reliable. The nasal bone appears as an echogenic line parallel to and thicker than the echogenic skin line overlying the nasal bridge. It is best seen when the footplate of the transducer is parallel to the long axis of the nasal bone. The two parallel lines of the nasal bone and skin line comprise the “equal sign” (Fig. 7.14). The nasal bone is considered absent if the deeper line is not present. Nasal bone assessment appears to be more difficult than NT assessment. Nevertheless, there are reports that, with adequate training and experience, assessment for presence or absence of nasal bone can be performed with success in up to 99 % of fetuses [80].