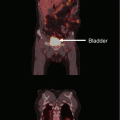
Fig. 1
Target volume concept according to ICRU 62: gross tumor volume (GTV); clinical target volume (CTV); internal target volume (ITV); planning target volume (PTV)
Table 1
Uncertainties in radiotherapy treatment planning and delivery
GTV stage | Inter- and intra-observer variability of target volume definition |
Sensitivity and specificity of imaging modality | |
PTV stage—intra-fractional | Patient motion |
Target motion due to: | |
Breathing | |
Heartbeat | |
Changes of the filling of hollow organs | |
PTV stage—inter-fractional | Patient setup: |
Rigid setup errors | |
Nonrigid setup errors | |
Shift of the target position due to: | |
Changes of the filling of hollow organs | |
Changes of the breathing pattern | |
Complex changes of the patients’ anatomy (e.g., atelectasis, effusions, etc.) | |
GTV regression/progression | |
Weight loss of the patient |
In recent years, multiple advanced technologies were introduced into radiotherapy treatment planning and delivery all owing the potential to reduce the margins described above resulting in reduced volumes of normal tissue exposed to mid- and high doses.
GTV Stage
Reduction of the GTV is certainly not possible, but modern imaging for target definition using, for example, MRI, SPECT, or PEt allows for more precise and reproducible definition of the recurrent cancer with especially improved differentiation between postirradiation or postsurgical fibrosis and active tumor.
CTV Stage
There are no data on the microscopic extension in the situation of loco-regional recurrences after a prior course of radiotherapy. However, it has been suggested, for example, for reirradiation of head-and-neck cancer, that the pattern of treatment failure after confining the target volume to the recurrent GTV with tight safety margins is in-field, which limits the potential benefit of elective nodal irradiation or irradiation of larger volumes where microscopic spread is assumed (Popovtzer et al. 2009). Additionally, the target volume concept needs to be adapted to the individual patient-specific situation: Different target volumes concepts can be considered in patients, where reirradiation intends short-term palliation or where a curative intend is followed.
PTV Stage, Intrafractional Uncertainties
Changes of the target position during the treatment fraction may have several reasons: patient motion, breathing motion, cardiac motion, peristaltic motion, and changes of the filling of hollow organs. Depending on the target location and depending on patient individual factors, the above listed uncertainties reach different magnitudes and the contribution of each factor to the total intrafractional uncertainty varies significantly. For example, breathing motion is the dominant uncertainty in the thoracic region but may vary from few millimeters to 3 cm between patients. Management of intrafractional motion is highly challenging because of the short timescale of these uncertainties (e.g., cardiac motion with >1 Hz) as well as the random, unpredictable nature of motion (e.g., patient motion).
PTV Stage, Interfractional Uncertainties
Uncertainties of the target position between treatment fractions influence safety margins significantly. The technique of stereotactic radiotherapy has been developed in the 1960s for high-precision radiotherapy of intracranial lesions (Leksell 1951, 1968), and this technique achieved an accuracy of patient setup with residual errors in the range of 1 mm. In the 1990s, the stereotactic principle of patient setup was transferred to the extracranial region, called stereotactic body radiotherapy (SBRT) (Lax et al. 1994). Recently, the need for external coordinates in stereotactic radiotherapy, both cranial and extracranial, has been questioned due to the availability of image guidance (IGRT) (Verellen et al. 2007), which allows verification of the target position prior to each treatment fraction. Besides changes of the target position, systematic changes of tumor volume and shape (regression or progression) and of the normal tissue (e.g., changes of pleural effusion and atelectasis; weight loss of the patient) have been observed during a fractionated course of radiotherapy. Adaptation of the treatment plan to these systematic changes in an adaptive feedback loop is currently a focus of research.
Choice of Irradiation Technique
It is important to adjust the irradiation technique to the individual patient case with location of the recurrent tumor, size and volume of the PTV, the type of normal tissue in relationship to the recurrence, and dose distribution of the previous treatment course being the most important factors. Kilo-voltage X-rays or electrons may be considered for superficial recurrences and brachytherapy in cases, where implantation of the catheters in a suitable geometry or intraoperatively is reasonable. The standard delivery methods for photon reirradiation are currently intensity-modulated arc techniques such as volumetric modulated arc therapy (VMAT) or RapidArc, which can provide more conformal dose distributions than 3D-conformal techniques (Stieler et al. 2011). Protons and heavy ions offer distinct physical and biological advantages over photons which allow to reduce the dose to normal tissue. Although these properties are of particular interest in the reirradiation situation, there is only limited data on the use of particle therapy for reirradiation published (Plastaras et al. 2014).
1.3 Safety Margins in Radiotherapy
Despite all technological progress, the clinical application of radiotherapy will never be without errors or uncertainties at the planning and delivery stage. Consequently, margins always have to be added to the CTV or GTV if adequate coverage of this target volume is intended. Most important is the differentiation between systematic and random errors (Fig.2). A systematic error affects all treatment fractions in an identical way and will result in a systematic difference between the intended and delivered dose distribution. An example is target delineation, where a certain part of the tumor is excluded from the target volume because of false-negative imaging for treatment planning. Random errors may affect all treatment fractions as well; however, all errors are centered around the planned position. It is the systematic error component which is most important and which should be minimized with highest effort in the primary and the reirradiation situation. The contribution of the random error component to the overall uncertainty and consequently to the overall safety margin is significantly smaller.


Fig. 2
Random (left) and systematic (right) uncertainties in radiotherapy treatment
The most commonly used margin concept is a population-based probabilistic concept: Application of a certain margin around the target volume ensures that the target volume is treated with at least 95 % of the prescribed dose in 90 % of the patient population (van Herk 2004). Systematic and random errors of all stages of radiotherapy need to be quantified for a patient population, and these data are used for calculation of population-specific safety margins. A different concept aims at adaptation of the safety margins to the individual, patient-specific uncertainties (Yan et al. 1997): Uncertainties are quantified at the beginning of the treatment course for each patient, and the safety margins and treatment plans are then adapted for the following treatment fractions based on the individual uncertainties (Fig.3).


Fig. 3
Work flow of patient individual adaptation of safety margins: (1) Start of treatment with population-based margins; (2) assessment of patient individual uncertainties; and (3) adaptation of safety margins to the patient’s individual errors
In the following of this chapter, we will focus in more detail on the distinct technological advances in external beam radiotherapy and discuss their potential role in the situation of reirradiation.
2 Imaging for Reirradiation
The integration of modern imaging modalities such as CT, MRI, and PET in the treatment planning process has become common practice; however, major advances in more specific imaging technologies have evolved in the last decade, requiring the radiation oncologist to have a detailed understanding of possibilities and limitations of these novel diagnostic modalities. Interdisciplinary discussion with radiologists or nuclear medicine specialists should lead to optimal integration of these modalities into the treatment planning process. Especially the development of image fusion software has significantly advanced in recent years, which was mainly driven by the radiooncological community; we are likely the specialty making greatest clinical use of image fusion, often more so than diagnostic radiologists themselves. Detailed discussion of imaging modalities for the different cancer sites is beyond the scope of this chapter and will be performed in the dedicated chapters of this book. Some important generalized points should be considered:
In the reirradiation situation, the radiation oncologist is frequently confronted with imaging results, which are significantly different to the situation of the primary irradiation (Meerwein et al. 2015): The normal anatomy is substantially altered after repeated surgical interventions and after prior radiotherapy. Especially differentiation between postsurgical/postradiotherapy scarring and recurrent tumor is difficult in many cancer sites: Our morphological imaging techniques of CT and standard MRI sequences are frequently limited in this situation. Additionally, we as radiation oncologists do not only need to differentiate between scarring tissue and recurrent tumor on a diagnostic level (yes or no) but must accurately delineate the recurrence in three dimensions for conformal treatment planning. The potential advantage of advanced imaging modalities in this situation will be demonstrated exemplarily in two cancer sites.
Malignant gliomas most frequently recur locally within a distance of about 2 cm to the primary lesion, which makes differentiation of recurrent cancer and posttherapeutic changes, especially radiation necrosis, difficult. Amino acid PET imaging in addition to standard MRI imaging was shown to increase sensitivity and especially specificity in diagnosis, grading, and determination of tumor extension of malignant gliomas (Pauleit et al. 2005; Hatakeyama et al. 2008). In the situation of recurrent malignant gliomas, amino acid PET imaging improved the accuracy for differentiation between radiation necrosis and recurrent tumor (Terakawa et al. 2008). Early clinical results suggest that integration of this biological information into target definition in primary radiotherapy and reirradiation of malignant gliomas alters the target volume in a significant proportion of the patients (Rieken et al. 2013; Munck Af Rosenschold et al. 2015; Lee et al. 2009). Additionally, amino acid PET uptake kinetics before reirradiation have shown to be of prognostic value (Niyazi et al. 2012), and the use of PET-based “biological” target volumes may even improve clinical outcome (Grosu et al. 2005) (Fig. 4).


Fig. 4
A 79-year-old patient was treated with standard radiochemotherapy (60 Gy and Temozolomide) to a left frontal glioblastoma. Twelve months later a local recurrence was surgically removed. While the surgeons reported gross total resection, post-OP MRI (within 2 days) showed residual tumor at the very frontal pole (left image). The hyperintense region at the posterior of the tumor cavity was attributed to blood. FET-PET however showed marked activity in the dorsal region while the frontal region was inactive (middle image). The contrast-enhanced planning CT shows no residual disease (right image). Yellow GTV MRI, Blue GTV PET, Red PTV surrounding both regions
After anterior resection or abdominoperineal resection for rectal cancer, the differentiation between fibrotic masses in the presacral operative bed and a local tumor recurrence is extremely challenging with conventional CT imaging (Lee et al. 1981). This requires the use of further imaging modalities for accurate target volume delineation for reirradiation: Magnetic resonance imaging of recurrent rectal cancer may help to determine infiltration into pelvic structures (Dresen et al. 2010), and dynamic contrast-enhanced imaging may predict R0 resection (Gollub et al. 2013). FDG-PET imaging was reported to allow differentiation between benign scarring tissue and a locally recurrent rectal cancer with high sensitivity and specificity (Ito et al. 1992), and combined PET/CT imaging was shown to further improve the accuracy by avoiding the misinterpretation of displaced pelvic organs as recurrent tumor (Even-Sapir et al. 2004). Integration of this functional imaging into radiotherapy treatment planning with a focal dose escalation in volumes of increased FDG-PET activity has been reported recently (Jingu et al. 2010).
In principle, the requirements for imaging in preparation for reirradiation are comparable to those necessary for high-precision radiotherapy in the primary setting. Here also, there should be no compromise in utilizing possibilities of treatment volume definition as both marginal misses and sequelae due to unnecessarily large treatment volumes are of special issue in the reirradiation situation.
3 Photon External Beam Radiotherapy
3.1 Conventional Two-Dimensional Radiotherapy
Conventional two-dimensional (2D) radiotherapy planning was the standard for decades in photon radiotherapy. Few radiation beams were selected, frequently directly opposing fields, three- or four-field arrangements. Size and shape of the fields were adjusted in 2D simulation X-ray images, and unless the tumor was visible in these planar images, filed shaping was mainly based on bony surrogates instead of the patient individual position, shape, and size of the tumor. Also visualization of normal structures was limited. This makes conventional 2D planning inappropriate for the majority of patients, where reirradiation is intended.
3.2 Three-Dimensional Conformal Radiotherapy
Three-dimensional conformal radiation therapy (3DCRT) has been the standard for most indications in photon radiation therapy in the last years, although it is increasingly replaced by intensity-modulated techniques.
It offers distinct advantages compared to conventional 2D radiotherapy, which are especially important in the reirradiation situation. Target volume definition is based on CT images, and coregistration of further imaging modalities like MRI or PET images is supported by all current treatment planning systems. This allows for more precise definition of both the target and critical organs-at-risk (OAR). These structures are visualized in the beam’s eye view for selection of the optimal beam directions and for field shaping aiming at best possible sparing of critical OARs. The benefit of 3D-CRT compared to 2D planning has been demonstrated in a randomized trial: In primary radiotherapy for prostate cancer conformal radiotherapy significantly reduced the incidence of proctitis and rectal bleeding compared to conventional radiotherapy; simultaneously local tumor control was not different between the two techniques (Dearnaley et al. 1999). This potential to reduced doses to the normal tissue with the consequence of reduced side effects is certainly of high clinical value in the reirradiation situation, where such large randomized trials are not possible (Fig. 5).
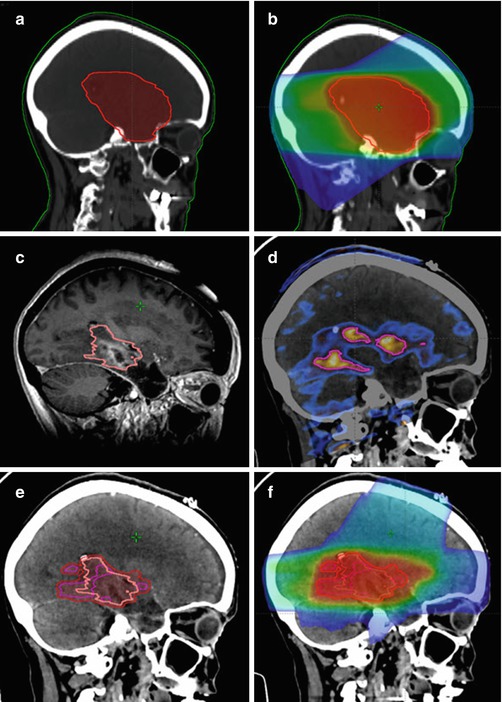

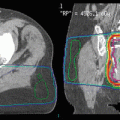
Fig. 5
Case example of reirradiation for a 58-year-old female patient with locally recurrent glioblastoma: Medical history: May 2013: primary diagnosis of glioblastoma; Macroscopically complete resection and adjuvant radiochemotherapy with 60 Gy and concurrent Temozolomide; No adjuvant chemotherapy due to grade III thrombopenia during radiochemotherapy November 2014: new contrast-enhancing nodule in the left temporal lobe; systemic therapy with bevacizumab May 2015: progressive recurrence in the left temporal lobe June 2015: repeat surgery, incomplete resection July 2015: stereotactic re-irradiation with 10 x 3.5 Gy using a PET/MRI-based target volume (a) Sagittal reconstruction of the primary volume definition; (b) Sagittal reconstruction of the primary irradition; (c) Target definition of the local recurrence in the MRI; (d)Target definition of the local recurrance in the FET-PET; (e) PET based GTV, MRI based GTV and PTV on the CT of the local recurrence; (f) Dose distribution for the local recurrent tumor
3.3 Intensity-Modulated Radiotherapy
The technique of intensity-modulated radiotherapy (IMRT) is an advancement of 3D-CRT. In 3D-CRT, homogeneous fluence profiles are delivered from each beam-angle. In contrast, IMRT is characterized by customized nonuniform fluence distributions to achieve certain dosimetric objectives (Fig. 6). 3D-CRT uses forward planning meaning that beams are specified, doses are calculated, and dose distributions in the relevant target volumes and OARs are evaluated at the end of the planning process. This is different to the inverse planning process in IMRT. Patient-specific dosimetric goals (objectives) are defined for all target volumes and OARs at the beginning of the treatment planning; the objectives are most frequently DVH parameters or since more recently biological parameters. These objectives are transferred into an IMRT optimization software, where the best possible beam parameters to achieve the desired dose distribution are calculated in an iterative fashion.


Fig. 6
3D-Conformal radiation therapy (left) and intensity- modulated radiotherapy (right) for a re-treatment of a lung metastasis. Non-uniform fluence distribution of the IMRT technique allows to more conformally irradiate the tumor and to better spare the organs at risk
Several techniques are commercially available for delivery of intensity-modulated radiation therapy. For conventional linear accelerators equipped with multileaf collimators, the static (step-and-shoot), the dynamic (sliding window), and rotational (volumetric/intensity-modulated arc therapy) techniques can be distinguished. The static step-and-shoot approach segments each IMRT field into a number of shaped subfields, and the sliding window technique modulates the fluence by moving the multileaf collimators (MLCs) while the radiation is being delivered to the patient. Both approaches achieve the energy fluence modulation by the MLCs, and the radiation is given from different static gantry angles. In contrast, volumetric/intensity-modulated arc therapy (IMAT/VMAT) rotates the linear accelerator around the patient while continuously delivering radiation, thereby applying hundreds of fields, by changing the position of the MLCs and the amount of radiation. A new promising approach still under investigation and not yet clinically available is the 4π- or dynamic-couch rotation technique, which combines the VMAT techniques with continuous rotation of the treatment couch (Smyth et al. 2013; Liang et al. 2015). Major advantages of these rotational techniques are significantly reduced delivery times as well as increased monitor units efficiency. A different IMRT solution is the tomotherapy approach, where the linear accelerator constantly rotates around the patient. The fluence modulation is achieved with a binary collimator, and fan beams are delivered in a CT-like “sliced” fashion, either in spiral or more recently in helical mode (Mackie et al. 1993).
Numerous planning studies have shown the potential of IMRT to generate highly conformal dose distributions, especially for complex, concave-shaped target volumes in close distance to organs-at-risk. In such cases, the sparing of normal tissue is significantly improved compared to 3D-CRT (Nakamura et al. 2014). The superiority or inferiority of one of the above described IMRT delivery techniques has been the issue of countless planning studies and is still highly controversial (Fig. 7). An analytical model was used by Bortfeld and Webb for comparison of TomoTherapy, single-arc VMAT, and static IMRT (step-and-shoot and sliding window IMRT), and they concluded that the TomoTherapy system has the greatest dose shaping flexibility at cost of decreased efficiency of the treatment delivery (Bortfeld and Webb 2009). However, it needs to be considered that despite these theoretical calculations and other planning studies comparing different IMRT hard- and software, the results of IMRT planning are dependent on the experience of the IMRT team (both physician and physicist) in terms of selection of optimization objectives for the inverse planning (Marnitz et al. 2015).


Fig. 7
Sliding window (left) and volumetric modulated arc therapy (right) treatment planning for re-treatment of a spinal metastasis. On the top the beam setups are shown, in the middle the dose distributions of the two techniques and on the bottom the dose volume histrograms
IMRT treatment planning, delivery, and quality assurance are in principle not different between a primary course of radiotherapy and the reirradiation course. However, some issues need to be considered more in detail in the reirradiation situation.
Unlike in 3D-CRT, IMRT planning distributes low doses over a larger volume of the patient. Additionally, volumes exposed to mid-doses or sometimes even high doses are frequently observed distant to the target volume. This may be of limited relevance in the primary course of treatment but could be deleterious in the reirradiation scenario, if these “hot spots” are located in volumes of normal tissue, where these additional doses exceed the radiation tolerance. Consequently, the physician should not only delineate the standard OARs as done in the primary treatment course; all volumes, where a significant prior irradiation dose had been delivered, should be defined as OARs and separate dose objectives should be defined for these volumes. Such normal structures could be the skin to avoid skin necrosis, joints and muscles to avoid contractures, and bones to avoid osteoradionecrosis.
In the reirradiation case, the radiation tolerance of normal structures is frequently significantly reduced. This is an extremely challenging situation for treatment planning, especially if this normal structure is located immediately next to recurrent cancer. A typical example is a spinal metastasis in the thoracic spine after primary radiotherapy for lung or esophageal cancer. In the situation of the OAR touching the PTV, the maximum dose of the OAR is the minimum dose to the PTV. The physician has now to decide where the dose gradient should be positioned: in the OAR aiming at a homogeneous dose in the PTV or in the PTV aiming at best possible sparing of the OAR at cost of an inhomogeneous dose in the target volume. The latter is certainly the most frequent situation in clinical practice. It is important that the IMRT planning objectives need to be adjusted to this desired dose distribution: Lower doses in the PTV immediately next to the OAR need to be allowed explicitly to the planning algorithm. The magnitude of this “underdosed” PTV depends on the steepness of the dose gradient between the target and the OAR. Mahan et al. reported a dose gradient of 10 %/mm using tomotherapy for retreatment of a spinal metastasis (Mahan et al. 2005). However, multiple variables influence the maximum achievable dose gradient: invariable factor like IMRT hard- and software and variable factors like geometry of target and OAR. For individual optimization of each plan, a ring-shaped help-volume around the OAR could be generated, where the dose gradient between OAR and PTV is to be located. Desired maximum and minimum doses of the OAR and the PTV excluding this help-volume are defined as hard constrains, and the size of the help-volume is step-wise decreased until these constrains can no longer be met by the planning system.
Clinical results of IMRT for reirradiation are promising. Loco-regional recurrent head-and-neck cancer is an example, where IMRT seems to improve outcome compared to conventional radiotherapy or 3D-CRT. Lee et al. reported about reirradiation in 105 patients with loco-regional recurrent head-and-neck cancer, and IMRT was used in 70 % of the patients (Lee et al. 2007). The median prior dose was 62 Gy and the median reirradiation dose was 59.4 Gy. Two-year loco-regional progression-free survival was 50 % and 20 % for patients treated with IMRT and non-IMRT, respectively. This benefit of IMRT remained statistically significant in multivariate analysis with a HR of 0.37. Other groups confirmed these favorable rates of ~50 % 2-year loco-regional control using IMRT (Biagioli et al. 2007; Duprez et al. 2009). Nevertheless, severe late toxicity was still considerable in these series.
Spinal metastases in previously irradiated areas are ideal IMRT indications for pain reduction or because of neurological symptoms (Fig. 8). Here, IMRT allows effective sparing of the spinal cord while treating the vertebral tumor, which is not possible with conventional radiotherapy or 3D-CRT. Milker-Zabel et al. reported the outcome in 19 patients with symptomatic spinal metastases, where a previous irradiation delivered a median dose of 28 Gy (Milker-Zabel et al. 2003). The median reirradiation dose was 39.6 Gy, while the dose to the spinal cord was limited to 20 Gy. With a median follow-up of about 1 year, only one patient developed a local recurrence. Pain relief and improvement of neurological deficits was achieved in 13/16 patients and 5/12 patients, respectively. No acute or late toxicity grade >II was observed. Further data are needed for confirmation of these promising results. Sterzing et al. reported on reirradiation of spinal metastases in 36 patients: The initial irradiation dose was 36.3 Gy on average, and after an interval of 17.5 months a dose of 34.8 Gy was delivered using TomoTherapy IMRT (Sterzing et al. 2010). Promising rates of pain reduction and local control were reported and no severe toxicity was observed.


Fig. 8
Case example of a reirradiation for a spinal metastasis in a 62-year-old male patient with metastatic prostate cancer. Medical history: 2010: primary diagnosis of localized prostate cancer; antihormonal therapy, rejection of local therapy. January 2015: locally invasive prostate cancer, several bone metastases including the thoracic spine; laminectomy Th3-6 and tumor debulking; and dorsal instrumentation Th1-Th8. March 2015: postoperative radiotherapy to residual tumor with 5 × 4 Gy. January 2016: local progression with epidural growth Th4-5; reirradiation with 10 × 3 Gy. (a) Spinal metastases with GTV (yellow) based on the MRI and PTV (red); (b) IMRT dose distributions with a total dose of 40 Gy to the PTV and a maximum dose of 15 Gy to the spinal cord; (c) image-guidance using cone-beam CT with superposition of planning CT and verification cone-beam CT before (left) and after (right) image registration
4 Three and Four Dimensional Treatment Plan Evaluation
If possible, the dose distribution of the first radiotherapy series should be available for treatment planning and plan evaluation. Information on maximum doses or DVH data of the first radiotherapy series is insufficient because of the missing spatial relationship to the current treatment. If this information about the previous dose distribution is not available, for example, because the patient had been treated with 2D conventional planning, this radiotherapy series should be resimulated in the current planning CT. However, one needs to be aware that the resimulation may not reflect the situation at the first irradiation course, because the patient’s anatomy could have changed, for example, due to the recurrent tumor or weight changes.
Three-dimensional dose distributions need to be evaluated carefully in terms of target coverage and especially in terms of normal tissue doses. DVHs are helpful tools for evaluation of the dose distributions, but one needs to be aware of the limitations of DVHs, where all spatial information is lost.
If the first treatment series was a 3D-CRT or IMRT irradiation and the treatment plan is digitally available, one could accumulate the dose distributions of the first and the current treatment series for a better risk assessment of the reirradiation. Accumulation of two dose distributions delivered at different times is called 4D dose calculation or 4D planning. Three important issues need to be considered for this 4D dose accumulation.
- 1.
Data about recovery of normal tissue and their modeling in treatment planning and evaluation are rare. Accumulation consequently simulates a worst case scenario without any recovery.
- 2.
Accumulation of physical doses would require conventional fractionation throughout the target and OARs, which is infrequently the case. Single-fraction doses different from 2 Gy should be weighted according to their biological effectiveness using the linear-quadratic model prior to dose accumulation. Calculation of 2 Gy-equivalent total doses (Lebesque and Keus 1991; Maciejewski et al. 1986) is an elegant method, resulting in numbers, which can be compared to tolerance doses for a single course of radiotherapy (Marks et al. 2010).
- 3.
The patient’s anatomy of the previous and the current treatment plan is certainly different, which makes 1:1 dose accumulation in the current CT data set misleading. A critical organ could have been displaced by the recurrent tumor, and this displacement of the critical organ in the current CT image relative to the situation of the first treatment course has to be considered in the process of dose accumulation (Fig. 9). Deformable registration between both image data may need to be performed and the resulting deformation map applied to the previous dose distribution: The deformed previous dose distribution and the current dose distribution are then accumulated and displayed in the current CT data set with the recurrent tumor and preset location of the relevant OARs (Jumeau et al. 2015). Several commercial solutions are nowadays available to perform such dose accumulations; however, methods to allow the user to evaluate the uncertainty of the deformable registration and subsequent accumulation are missing. Therefore, residual dose distributions, especially in situations with large anatomical change, have to be evaluated carefully (van Rijssel et al. 2014).

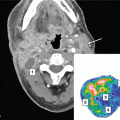
Fig. 9
Illustration of a recurrent skull base tumor (red), which causes a displacement of the right optical nerve (blue). Accumulation of the dose to this optical nerve from both irradiation series needs to account for this displacement by means of deformable image registration (indicated by vectors)
5 Stereotactic Radiotherapy and Image Guidance
In the reirradiation situation, the target volume is usually limited to the recurrent macroscopic tumor without extensive elective CTV margins in order to reduce normal tissue exposure (Mantel et al. 2013). Stereotactic intracranial radiotherapy and stereotactic body radiotherapy (SBRT) in combination with image guidance (IGRT) provide an accurate means of highly conformal treatment delivery and patient positioning which can further spare organs-at-risk during reirradiation (Guckenberger et al. 2014).
Patient setup for daily radiotherapy has traditionally been performed by alignment of the room lasers with patient skin marks. This procedure assumes that there is a fixed, rigid relationship between the skin marks and the actual target volume. However, this method of patient setup is one of the major uncertainties in the radiotherapy delivery process contributing significantly to the safety margins (Hurkmans et al. 2001). Patient-specific uncertainties are imperfect alignment of the patient to the laser, mobility of the skin relative to the bony anatomy, and mobility of the tumor relative to the bony anatomy. These setup errors are especially important for treatment plans with steep dose gradients between the target and the organ-at-risk: For IMRT treatment of spinal metastases, it has been shown that patient setup errors as small as 1 mm can increase the dose to the spinal cord by a clinically relevant amount (Guckenberger et al. 2007a) (Fig. 10). Consequently, highly conformal treatment plans using IMRT or Protons pose a significant risk of target underdosage and/or OAR overdosage unless precise patient setup is ensured.


Fig. 10
Effect of set-up errors on dose to organ-at-risk: Upper left image: Dose distribution in the axial plane in VMAT plan for a spinal metastasis. Upper right image: Simulation of patient set-up error with a lateral shift of 5 mm to the left Lower image: Dose to the spinal cord: The yellow DVH curve displays the prescribed PTV dose according to the treatment plan. The turquoise DVH curves are dose distributions to the spinal cord resulting from simulated set-up errors. DVH curves for the esophagus and GTV are shown in lilac and pink, respectively
The stereotactic technique has been proven as highly effective for accurate patient setup. Stereotactic radiotherapy has traditionally been defined by a system of external coordinates. This stereotactic system is rigidly fixated to the patient and forms the basis for treatment planning with definition of the isocenter position and patient setup before treatment. In the cranial region, this has been traditionally practiced in an invasive fashion, where the stereotactic frame is fixated to the patient’s skull. This offers best accuracy of patient setup; however, the invasiveness of the procedure requires planning and treatment finished within 1 day by means of radiosurgery. Noninvasive techniques for fractionated regimes were developed using thermoplastic mask or bite-block systems; the tradeoff to perform a fractionated treatment courses was a slightly reduced accuracy of patient setup. Initially developed for intracranial treatments, the stereotactic technique has been successfully adopted for extracranial stereotactic radiotherapy (Fig. 11).


Fig. 11
Stereotactic patient setup: Cranial stereotactic radiotherapy with invasive fixation of the stereotactic ring (a) and the attached stereotactic frame with the system of external coordinates (b). Thermoplastic mask used in image-guided stereotactic radiotherapy (c). Stereotactic body radiotherapy using the Stereotactic bodyframe with a customized vacuum cushion (d) and a system of external coordinates (e)
Recently, image-guidance techniques have been developed, which are located in the treatment room and allow for daily verification of the patient setup with online correction of setup errors before the start of treatment. It has been shown that these IGRT techniques are at least equivalent in terms of patient setup accuracy compared to invasive frame-based stereotactic radiosurgery in the cranial region (Ramakrishna et al. 2010). The accuracy of patient setup for fractionated stereotactic radiotherapy in the cranial (Guckenberger et al. 2007b) and extracranial region (Guckenberger et al. 2006) is improved with IGRT compared to frame-based stereotactic patient positioning. Additionally, sufficient soft-tissue contrast in these verification images or implantation of radio-opaque markers make verification of the actual tumor position possible, which is important for targets, where mobility independent from bony anatomy has been described (Fig. 12). A treatment using daily image guidance with online correction of setup errors for high-precision radiotherapy is considered as “frame-less stereotactic radiotherapy”: The stereotactic frame is replaced by image guidance with the patient’s image as the “system of coordinates” for isocenter localization (Haertl et al. 2013). The available technologies of IGRT are summarized in Table 2.