Fig. 1
(2-[18F]fluoroethyl)-l-tyrosine
Despite all efforts, the success of utilizing radiolabeled amino acids to measure protein synthesis in vivo is still limited or unsatisfactory. The difficulties in utilizing radiolabeled amino acids for in vivo determination of protein synthesis are due to the wide variety of possible metabolic pathways of amino acids (Haubner 2010).
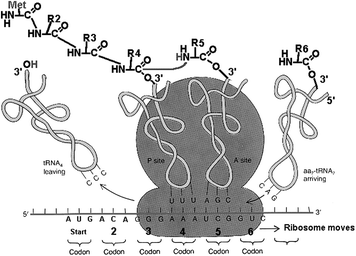
Puromycin has played an important role in our understanding of the eukaryotic ribosome and protein synthesis. It has been known for more than 40 years that this antibiotic is a universal protein synthesis inhibitor that acts as a structural analog of an aminoacyl-tRNA (aa-tRNA) (Yarmolinsky and Haba 1959) in eukaryotic ribosomes (Fig. 2). Nathans (1964) demonstrated that the eukaryotic ribosome mistakenly inserts puromycin in place of aa-tRNA, resulting in truncated proteins containing the drug at their C-termini. In a later examination of this mechanism, Miyamoto-Sato et al. (2000) concluded that puromycin inhibits translation solely through C-terminal labeling of peptides and not through additional pathways. This classic model for puromycin action admits only a single mode of action, i.e., covalent attachment to the nascent peptide chain (Starck and Roberts 2002).
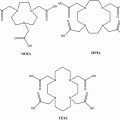
Puromycin–deoxycytidine conjugates (puromycin–dC conjugates) labeled with biotin have been utilized by molecular biologists to determine in vivo protein synthesis during the last years (Miyamoto-Sato et al. 2000; Starck and Roberts 2002; Nemoto et al. 1999). Their metabolism has been proven to be only involved in protein synthesis mimicking aa-tRNA. We decided to replace biotin, which is used as fluorescent dye for detection, with the chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) (Fig. 3). Both molecules, biotin and DOTA, are inserted to the puromycin precursors as activated N-hydroxysuccinimide (NHS) esters. In this context, positron-emitting radio metals such as 68Ga can be used for labeling.
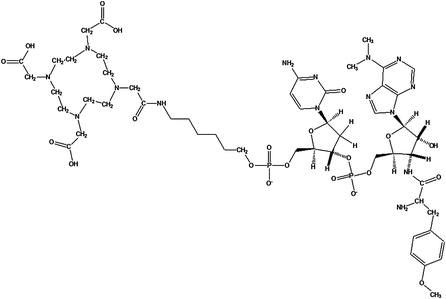

Fig. 3
DOTA-NHC6-dC-puromycin: DOTA-NHC6-deoxycytidine-puromycin was produced at Purimex (Grebenstein, Germany), analogous to the biotin derivatives developed by Starck and Roberts (2002) (puromycin-2P)
Consequently, the purpose of this study is to investigate whether 68Ga-labeled puromycin-based DOTA conjugates can be utilized for imaging of protein synthesis in vivo and to demonstrate the differences between natural amino acids and their fluorinated analogs in metabolic pathways involved in protein synthesis utilizing [3H]tyrosine (representing natural amino acids) and 2-fluoro-[3H]tyrosine as its fluorinated analog.
2 Methods
2.1 Equipment and Reagents
DOTA-Pur (Fig. 1) was purchased from Purimex (Grebenstein, Germany). [3H]tyrosine and 2-fluoro-[3H]tyrosine of the highest purity available were purchased from Moravek (Bera, USA) or Amersham Biosciences (Hammersmith, UK). Chelex 100 in sodium form and human serum albumin (HSA) were purchased from Sigma (Steinheim, Germany). All other reagents were purchased from Merck (Darmstadt, Germany) or Fluka (Steinheim, Germany) with the highest purity available. Ultrapure water (Aquatec Water Systems, Inc., CA, USA) was used for all procedures. To avoid metal contamination, all solutions used in conjugation and radiolabeling reactions were passed through a Chelex 100 column (1 × 10 cm). All glassware was washed with 2 M HCl and ultrapure water.
2.2 68Ga Production
68Ga was produced from a commercially available 1 110-MBq 68Ge-68Ga generator based on a TiO2 phase adsorbing 68Ge4+ (Cyclotron Co. Ltd., Russia). Generator-eluted 68Ga is preconcentrated and purified using miniaturized column with organic cation exchanger. The method used for separation of 68Ga from 68Ge is the most effective method to eliminate 68Ge breakthrough and the generator column material contaminants from the product, and was developed by Zhernosekov et al. (2007) and published previously.
2.3 Synthesis of DOTA-NHC6-dC-Puromycin (DOTA-Pur)
DOTA-Pur was synthesized using a puromycin-tethered CPG support by the usual protocol for automated DNA and RNA synthesis [phosphoramidite method (Starck and Roberts 2002)] following our design by Purimex (Grebenstein, Germany).
2.4 68Ga-Labeling of DOTA-Pur
DOTA-Pur (50 μl) in H2O (1.11 nmol/μl) was added to 30 MBq 68Ga in 100 μl 0.2 M HCl and incubated in a heating block at 90°C for 20 min while shaking. The reaction mixture was purified on a reversed-phase C-18 cartridge (Strata-X 33 μm Polymeric Sorbent, 60 mg/mL; Phenomenex, Inc., USA). After transfer to the cartridge, the product was washed with 2 mL H2O. 68Ga-DOTA-Pur was eluted from the resin in 300 μl ethanol. The ethanol was then evaporated and the product dissolved in 0.9% saline to a final volume activity of 30 MBq/ml.
2.5 In Vitro Studies
All described in vitro studies were carried out in DU145 (HTB-81™, human prostate carcinoma) and BJ (CRL-2522™, human foreskin fibroblasts) cells. Cells were purchased between 2 and 4 months before conducting the experiments. Cells are commercially available at LGC/ATCC, Lomianki, Poland.
2.6 In Vitro Uptake and Protein Incorporation
DU145 cells were routinely cultivated in standardized supplemented full media [RPMI1640 + 10% fetal calf serum (FCS) +1% penicillin/streptomycin (PAA, Czech Republic)] at 37°C in a humidified atmosphere containing 5% CO2. Cell preparation for the experiments was carried out 48 h beforehand, seeding 2 × 105 living cells per well in 6-well plates (Becton, Dickinson and Company, USA) using 2 mL normal media. Therefore, cells were harvested by trypsinization, and the number of living cells was determined utilizing a CASY TT cell analyzing system (Innovatis, Reutlingen, Germany). During the experiments, the number of living cells was counted for each measurement using CASY TT. All data were normalized to 1 × 106 living cells.
Total cellular uptake was measured after incubation of the cells with 84 pmol of tracer (68Ga-DOTA-Pur, [3H]tyrosine, or 2-fluoro-[3H]tyrosine) per well for 2 h. Incubation was stopped by discarding the treatment media and washing the cells twice with ice-cold PBS. In the next step, cells were dissolved in 500 μl lysis buffer [10% NaOH + 5% sodium dodecyl sulfate (SDS)], and the total sample activity was measured in a fully automated gamma counter (2,470 Wizard; Perkin Elmer, USA). For measuring protein incorporation, proteins were then precipitated and isolated by addition of trichloroacetic acid (TCA) to final concentration ≥10%. After centrifugation, the supernatant was removed and the precipitate washed twice with TCA solution. Activity contained in the acid solution and protein pellet were determined in the automated gamma counter. Results were calculated using GraphPad Prism software (GraphPad Software, Inc., CA, USA).
2.7 Inhibition of 68Ga-DOTA-Pur, [3H]Tyrosine, and 2-Fluoro-[3H]tyrosine Incorporation into Proteins
Experiments using 3-[2-(3,5-dimethyl-2-oxocyclohexyl)-2-hydroxyethyl] glutarimide (cycloheximide) (Fluka, Steinheim, Germany) as noncompetitive inhibitor of protein synthesis were carried out in 6-well plates (Becton, Dickinson and Company, USA) with 2 × 105 cells/well inhibitor. Cycloheximide exerts its effect by interfering with the translocation step in protein synthesis (movement of two tRNA molecules and mRNA in relation to the ribosome), thus blocking translational elongation.
DU145 and BJ cells were treated simultaneously with 84 pmol/well tracer (68Ga-DOTA-Pur, [3H]tyrosine, or 2-fluoro-[3H]tyrosine) and inhibitor (cycloheximide) concentrations between 10 pmol/well and 20 nmol/well. Cells were incubated for 1 h, and total cellular uptake and protein incorporation were measured as described above. Results were calculated as response in percent of noncompeting uptake and plotted semilogarithmically against inhibitor concentration using GraphPad Prism software (GraphPad Software, Inc., CA, USA).
2.7.1 68Ga-DOTA-Pur μPET Imaging in Tumor-Bearing Rats
All used cell lines and animals were provided by the Institute of Physiology, Johannes Gutenberg-University Mainz, Germany. Rat cell lines AT1 (subline of Dunning R3327 rat prostate carcinoma) and Walker carcinoma 256 (subcutaneous solid tumor) were used in all experiments. AT1 cells were grown in RPMI medium supplemented with 10% FCS and Walker cells in DMEM (Gibco No. 430–1,600; Gibco, Darmstadt, Germany) containing 5% FCS, 2.5 μg/mL amphoterin B, 50 μg/mL kanamycin, 100 μg/mL streptomycin, and 100 U/mL penicillin at 37°C under a humidified 5% CO2 atmosphere. Medium was changed routinely every 2 days, and passages were done once a week.
For tumor implantation with AT1 cells and Walker cells, male Copenhagen rats (Charles River Wiga, Sulzfeld, Germany; body weight 150–200 g) and male CD(SG)IGS rats (Charles River Wiga, Sulzfeld, Germany; body weight 150–200 g) were used, respectively. Animals were housed in the animal care facility of the Johannes Gutenberg-University Mainz and allowed access to food and water ad libitum before the investigation. All experiments had previously been approved by the regional Animal Ethics Committee and were conducted in accordance with the German Law for Animal Protection and the UK Coordinating Committee on Cancer Research (UKCCCR) Guidelines. Solid carcinomas of both cell lines were heterotopically induced by injection of the cells (0.4 ml, 104 cells/μL) subcutaneously into the dorsum of the hind foot. Tumors grew was flat, solid spherical. Volumes were determined by measuring the three orthogonal diameters (d) of the tumors and using an ellipsoid approximation with the formula: V = (π/6) × d 1 × d 2 × d 3. Animals were used for μPET studies when the tumors reached volumes between 1.0 and 2.0 mL, approximately 10–14 days after tumor cell inoculation.
μPET imaging was performed on a μPET Focus 120 small animal PET (Siemens/Concorde, Knoxville, TN, USA). During PET measurements, anesthetized (3% isoflurane in oxygen) animals (230–270 g body weight) were placed tail first supine in the field of view. To keep the body temperature of the animals stable, an infrared light source was placed near the animals. 68Ga-DOTA-puromycin (20–25 MBq) in 0.7–0.9 ml 0.9% NaCl solution was administered as a bolus via a previously inserted catheter in the tail vein while the animals were already placed inside the μPET.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree