Marcus Presley • Daniel B. Brown
Hepatocellular carcinoma (HCC) remains a uniformly lethal disease process in the absence of curative procedures such as transplantation or resection. Okuda et al.1 described a median survival of 1.6 months for untreated patients in 1984. The dismal prognosis for patients with HCC is largely unchanged, with a median survival of 3 months reported in a prospectively evaluated group in 2005.2 For decades, no systemic therapies were available and resection remains largely infeasible given the nearly universal presence of cirrhosis. In 1975, a series of 14 patients treated with systemic doxorubicin for HCC with 3 responders was reported.3 A similar 16.6% response rate was identified in a 42-patient cohort in 1977.4 Intra-arterial doxorubicin was then studied, and when infusion was performed with embolization, survival was extended to 19 months.5,6 These reports spurred interest in further development of chemoembolization of HCC.
Several randomized trials were performed in the 1990s without evidence of benefit from chemoembolization. These studies had various flaws in their construction, including treatment of patients at timed intervals rather than based on imaging findings, poor patient selection, limited sample size unlikely to demonstrate statistical benefit, and inappropriate choice of embolic agents such as coils which limited the ability to retreat patients.7–10
In 2002, two prospective randomized trials were published demonstrating that in appropriately selected patient populations, chemoembolization resulted in superior survival compared to symptomatic treatment. Lo et al.11 randomized 80 patients to either chemoembolization with cisplatin, Lipiodol, and gelfoam or best supportive care with survival as the primary end point. Patients were carefully selected with exclusion of individuals with advanced cirrhosis or macroscopic vascular invasion. Survival rates at 1, 2, and 3 years following chemoembolization were 57%, 31%, and 26%, respectively, compared to 32%, 11%, and 3% for supportive care (P = .002). Llovet et al.12 performed a three-arm trial comparing chemoembolization, bland embolization, and best supportive care in a total of 112 patients with a primary end point of survival. The trial was halted when the chemoembolization group reached superiority versus supportive care with 1- and 2-year survivals of 82% and 63%, respectively, compared to 63% and 27% (P = .025). The outcomes from these two studies lent significant credibility to the practice of chemoembolization.
DEVICE/MATERIAL DESCRIPTION
Oily chemoembolization is one of the most cost-effective treatment options in all of interventional oncology. The cost of the chemotherapy, Lipiodol, and embolic is much less than drug-eluting beads or yttrium 90 (90Y) microspheres. A challenge when comparing outcomes from different studies is the tremendous variation in embolics, chemotherapeutic agents, and how these tools are combined in a given procedure. This issue is further clouded by the absence from the marketplace of some of the widely reported agents over the last decade. At various times, Ethiodol, powdered cisplatin, and powdered doxorubicin have been unavailable. As a result of the mentioned variables, standardization of chemoembolization has been difficult to achieve.
The embolic agent of choice varies by study. In the previously mentioned randomized trials of Lo et al.11 and Llovet et al.,12 gelfoam was the embolic agent. Other authors have used polyvinyl alcohol with a transition to use of calibrated embolic microspheres as they became available.13–15 However, direct comparisons of outcomes in oily chemoembolization with different embolic agents are sparse. One review compared gelfoam powder with polyvinyl alcohol and Ethiodol and resulted in nearly identical survival: 519 ± 80 days for gelfoam powder versus 511 ± 75 days for polyvinyl alcohol (P = .93).16
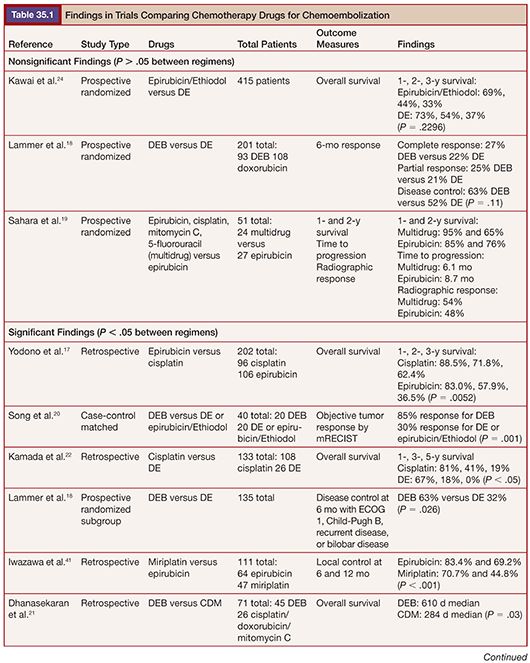
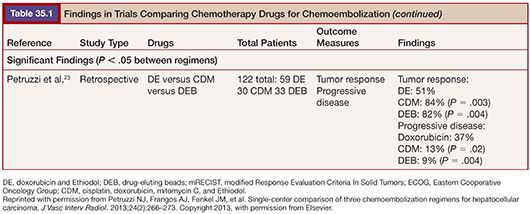
Literature comparing different chemotherapy regimens is more plentiful. Of note, no prospective randomized trial has identified a statistically significant difference in targeted outcomes when comparing regimens17–23 (Table 35.1). One prospective study compared epirubicin to doxorubicin without any difference in survival identified at 1, 2, or 3 years.24 Given the intermittent unavailability of powdered doxorubicin, this finding suggests that epirubicin could be an adequate alternative.
A final consideration is timing the addition of embolics to the chemotherapy/oil mixture. A comparison of various approaches was compared by Geschwind et al.25 They studied the percentage of intended chemotherapy that could be infused as well as the subsequent arterial patency in a treated area. Patients received one of three regimens: chemotherapy, oil, and polyvinyl alcohol particles simultaneously; chemotherapy and oil followed by polyvinyl alcohol; or chemotherapy and oil followed by gelfoam. When the embolic was mixed simultaneously with the chemotherapy and oil, both the intended percentage of chemotherapy to be infused as well as the subsequent arterial patency was lower than when embolics were added following oil/chemotherapy infusion. Outcomes for polyvinyl alcohol and gelfoam were similar regarding percentage of chemotherapy delivery (75.3% polyvinyl alcohol vs. 80.6% gelfoam) and subsequent arterial patency (74% polyvinyl alcohol vs. 81% gelfoam).
The delivery equipment is relatively standard for interventional radiology laboratories and includes arterial sheaths, selective catheters, and microcatheters. For patients with HCC, selection of segmental and subsegmental arteries is typically performed, making microcatheters a necessity for most, if not all, procedures. Microcatheters with 0.027-in lumens can perform diagnostic angiography as well, tolerating power injection rates up to 4 mL per second. The ability to perform high-quality angiography via the microcatheter is supplemented by the expanding use of C-arm computed tomography for targeting in this patient population. The viscosity of the chemotherapy and oil can make injection through smaller lumen catheters challenging, another reason to use larger bore devices. The mixture of oil and chemotherapy is toxic and can melt the junction of standard stopcocks and syringes with potential leakage of contents. At the advent of the procedure, glass syringes and metal stopcocks were used to avoid this issue. Currently, we use syringes and stopcocks made of polycarbonate. Although polycarbonate has made chemoembolic suspension less cumbersome, there is still potential for leakage and operators should operate with great care.
TECHNIQUE
Preprocedure Considerations
Patients should have either a dynamically enhanced computed tomography (CT) or magnetic resonance (MR) scan within 4 weeks of treatment. We hydrate patients with approximately 100 mL per hour of normal saline unless there is a history of congestive heart failure or other cardiac disease in which case lower rates are given. Patients are given midazolam and fentanyl for moderate sedation and ondansetron to control nausea. Labs are reviewed the morning of the procedure. When treating HCC, elevated bilirubin is a common scenario secondary to underlying cirrhosis. Historically, patients with total serum bilirubin greater than 2 mg/dL were approached with great caution. With a superselective approach, treatment of a small portion of the liver is possible, allowing for safe treatment in patients with liver dysfunction. Thrombocytopenia is also commonly present in cirrhotics as a consequence of portal hypertension. We do not routinely transfuse platelets as consumption by the engorged spleen will frequently limit the response. Preprocedure antibiotics are not required in patients with intact biliary anatomy (see complications section).
Procedural Considerations
Access to the femoral artery is obtained using the Seldinger technique, and a 6-Fr sheath is placed and connected to a pressurized flush. A 5-Fr selective catheter is used to select the superior mesenteric and celiac arteries and diagnostic arteriography performed. With advances in cross-sectional imaging, aberrant anatomy is frequently identified preprocedure that allows for targeted angiography with smaller volume contrast injections. Following selective angiography of the mesenteric artery, subselection with a microcatheter is performed over a hydrophilic microwire. Repeat digital subtraction angiography (DSA) is performed at the lobar or segmental level depending on the tumor location. If there is concern regarding complete tumor coverage in the selected area, we will perform C-arm CT with the reconstructed images compared to the preprocedure imaging. Once we confirm that the targeted area has been selected, the oil and chemotherapy mixture is suspended via a three-way stopcock. We use 1 mL of oil for each centimeter of tumor diameter up to 15 mL. Following injection of 4 to 6 mL of intra-arterial lidocaine, the suspended mixture is slowly infused using 3-mL syringes under fluoroscopic guidance, ensuring that antegrade flow is maintained. Once the entire mixture is infused, gelfoam slurry is added until the segmental artery reaches near stasis. If stasis is achieved before infusion of the entire slurry of chemotherapy, the gelfoam is added at that time. Final DSA is performed to evaluate for residual tumor enhancement. Our preference is to use closure devices in this patient group to decrease recumbency time due to postprocedural nausea and vomiting and the common incidence of thrombocytopenia. In our experience, success rates with closure devices are not adversely affected in the presence of thrombocytopenia.
Postprocedure Considerations
Patients are admitted overnight for hydration and pain control. Empiric ondansetron (8 mg) is given every 8 hours and a patient-controlled analgesia (PCA) pump is ordered, using Dilaudid 0.1 mg demand dose every 6 minutes without a basal rate. This level of analgesia is sufficient for over 90% of patients. Patients are started on a clear diet that is advanced as tolerated. Labs are rechecked overnight to ensure liver functions remain stable. The key components to discharge the next morning are the ability to tolerate oral intake and pain control with oral medications. During morning rounds, the PCA usage and patient pain scale is assessed. The PCA is discontinued if appropriate and the patient can be discharged following breakfast. We provide instructions on maintaining hydration following discharge and patients are asked to call interventional oncology if they are struggling with oral intake. Many times, early intervention with intravenous fluids in the clinic can avoid readmission. Patients are discharged with oral pain medication when appropriate as well as antiemetics. An appointment is made for follow-up imaging 4 weeks from treatment to assess treatment response.
CLINICAL APPLICATIONS
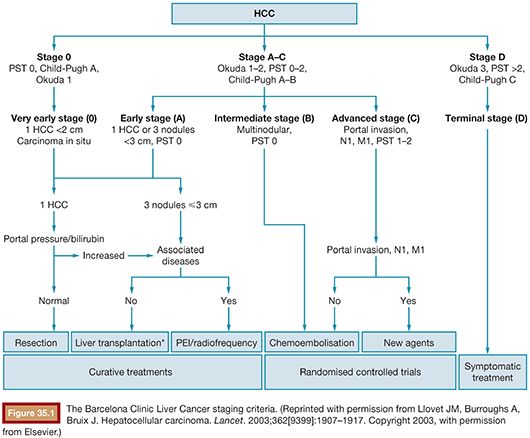
Chemoembolization is one of the most widely performed procedures for HCC because most patients present with cirrhosis too advanced to consider resection. Additionally, the number of patients requiring liver transplantation far outstrips the available supply of organs. To separate patients by disease status and prognosis, the Barcelona Clinic for Liver Cancer (BCLC) guidelines (Fig. 35.1) have been developed.26 In a review of over 1,700 patients, BCLC was a better predictor of survival than both alternative scoring systems and serum biomarkers.27 Survival was also relatively predictable by BCLC stage, with 5-year survival decreasing significantly with each advance in stage (Fig. 35.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree