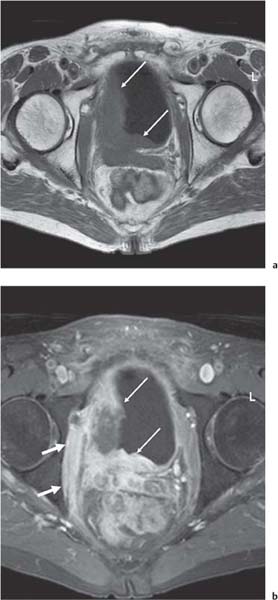
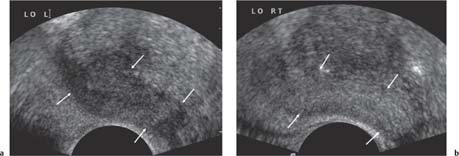
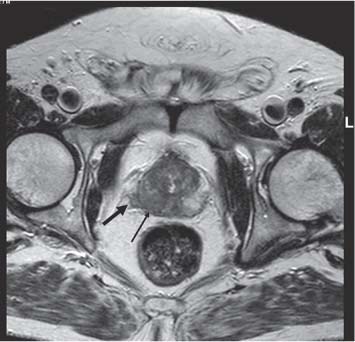
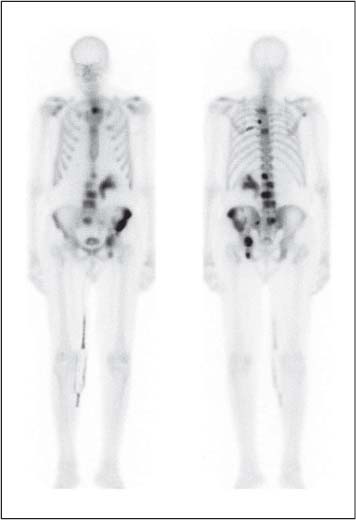
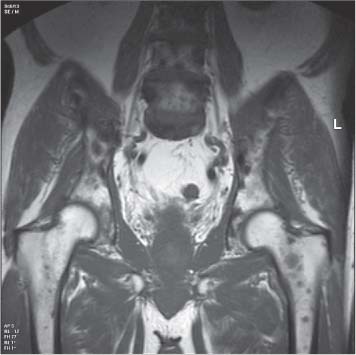
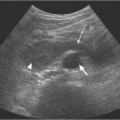
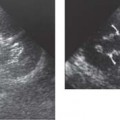
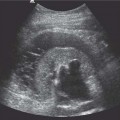
13 Oncological Management of Tumors of the Urogenital Tract The treatment of all tumors affecting the entire urogenital tract is an extensive subject and cannot be covered in a relatively short chapter such as this. We have therefore limited the discussion to the treatment of the four most common neoplasms, i. e., prostate, bladder, kidney, and testis. Increasingly, such neoplasms are treated in purpose-designed multidisciplinary clinics where patients can benefit from the expertise of specialist urological surgeons, as well as clinical and medical oncologists with a special interest in the treatment of genitourinary tumors. In addition, specialist urological nurses have an increasing role in patient management, providing information and support to patients and their families at what can be a difficult and stressful time. Discussion of the pathological features and radiological findings is an essential aspect of overall patient management and should ideally be undertaken in every patient diagnosed with a genitourinary neoplasm. For the purpose of discussion, bladder cancer treatment is best divided into the treatment of superficial and muscle-invasive disease. Superficial disease includes those tumors which are noninvasive or invade only subepithelial tissue. In the United Kingdom, this disease is usually treated by urologists. Tumors invading into bladder-wall muscle and beyond are usually treated by oncologists (Fig. 13. 1). Fig. 13.1 a Axial T1-weighted magnetic resonance imaging (MRI) scan showing an extensive bladder tumor (arrows) involving the posterior and right lateral wall of the bladder with clear evidence of extravesical extension into the perivesical tissues. b Gadolinium-enhanced image of the same area showing the extensive primary lesion (arrows) with an area of central tumor necrosis. In addition, however, there is better delineation of the extravesical extension and marked contrast enhancement of the right obturator internus muscle (thick arrows) indicative of tumor involvement There are a number of prognostic factors associated with superficial disease, including the total number of tumors, histological grade, tumor size, and recurrence at three-month cystoscopy.1,2 Low-grade papillary tumors can be treated by transurethral resection (TUR) alone or by thermocoagulation with a neodymium laser. Random biopsies of apparently healthy mucosa are also taken, as there can be a field change affecting the entire urothelium. This therapy is given in order to either prevent recurrence following endoscopic resection or as definitive local therapy for unresectable superficial disease. A number of agents are available, including adriamycin, epirubicin, and mitomycin C. There is little to choose between these drugs in terms of efficacy and toxicity. A meta-analysis of randomized trials using this treatment showed that the disease-free interval could be prolonged with intravesical chemotherapy, but there was no impact on progression-free or overall survival.3 Patients with adverse prognostic factors are usually advised to undergo a course of treatment although the most effective regimen is still undecided. Even a single installation given in the immediate postresection phase can be effective.4 A common regimen is to prescribe mitomycin C or epirubicin weekly for six weeks and then reassess. There is little or no systemic toxicity with such treatment, but 6–40% of patients will develop symptoms secondary to chemical cystitis. Patients with papillary noninvasive lesions can expect a five-year survival of 90%. This falls to 75% for T1 lesions. Superficial tumors of high grade are a distinct pathological entity with progress to muscle invasion in 50% of cases.5 Carcinoma in situ (CIS) also has an aggressive course and is treated in a similar way to high-grade superficial disease. Intravesical therapy is usually given first, with radical cystectomy reserved for chemotherapy failure. There is no effect on overall survival treating in this way.6 A course of bacille Calmette–Guérin (BCG) therapy is given in these cases, often weekly for six weeks followed by reassessment. The mechanism of action of BCG is largely unknown but it is known to have immunomodulatory effects and also induces a major inflammatory reaction within the bladder mucosa. The response rate to this treatment is 70%. However, only about 30% remain tumor-free at 10 years.7 Following staging investigations to exclude distant metastases, patients considered to have localized bladder cancer can be offered either radical cystectomy or nonsurgical treatment with radiation or chemoradiation. Distant metastases are sought by means of chest xray (CXR), computed tomography (CT) of the abdomen and pelvis, magnetic resonance imaging (MRI) of the pelvis, and bone scanning if appropriate. Factors to be considered in guiding patients in their decision include the following: • The local extent of the tumor as indicated by cystoscopy and bimanual examination • The local appearances on CT or MR imaging • The presence of pelvic lymphadenopathy • The general medical condition of the patient The presence of poor prognostic factors, e. g., anemia or hydronephrosis • The wishes of the patient • The pathological features, e. g. adenocarcinoma responds poorly to radiation • The presence of CIS in random bladder biopsies Radical cystectomy involves complete removal of bladder, prostate, and seminal vesicles in the male. In females, the corresponding operation involves removal of the bladder, cervix, uterus, fallopian tubes, and anterior vaginal wall. Urine is then diverted into one of three reservoirs: • Ileal conduit where both ureters are implanted into the terminal ileum and brought out as an incontinent stoma • A continent reservoir where an intra-abdominal reservoir is fashioned from the stomach or bowel and urine drained by regular catheterization8 • Orthotopic bladder substitution where a similar reservoir is reanastamosed to the urethra9 The urethra should not be spared if the tumor involves the bladder neck or posterior urethra or in the presence of CIS. The five-year survival rate for patients treated by cystectomy alone is between 15 and 80%, depending on staging. Retrospective surgical results are generally better than for radiation alone, but when allowance is made for confounding factors such as selection bias, stage migration, i. e., clinical versus pathological staging and prognostic factors, there is little difference in overall results and certainly no survival benefit. Cystectomy can also be offered in the event of local failure following radiotherapy, again with no effect on overall survival. Nonsurgical options for localized or locally advanced disease include radiation alone, neoadjuvant and adjuvant chemotherapy, and concomitant chemoradiation. Modern radiotherapy techniques utilize planning CT scans and carefully fractionated regimens to minimize toxicity and avoid the chances of a geographical miss on the tumor. Prior to treatment the bladder is emptied to decrease the volume. Treatment is usually given in small daily fractions for four to six weeks to a total dose of 52–60 Gray (Gy). The side effects of radical radiotherapy include radiation cystitis, proctitis, and lethargy. In the longer term urinary function may deteriorate due to bladder fibrosis, although this is not common. Attempts have been made to improve the results of local treatments by the integration of chemotherapy. The recognition that a significant number of patients die of metastatic disease without local failure made the introduction of systemic therapy attractive in this setting. There have been a number of trials of chemotherapy given in a neoadjuvant setting, i. e., before definitive local therapy. The largest study reported to date is the MRC/EORTC Trial which examined the effect of three pulses of cisplatin-based chemotherapy before either cystectomy or radiation therapy. The survival benefit reported did not reach statistical significance, nor did the improvement in local control.10 However, a recently published meta-analysis of neoadjuvant chemotherapy in bladder cancer has shown a 5% overall survival benefit for the use of multiagent cisplatin-based therapy irrespective of the type of local treatment.11 Chemotherapy given after surgery or radiation, i. e. in the adjuvant setting, has been less well-studied and no definite conclusions can be drawn at this time. Chemotherapy agents can be utilized as radiation sensitizes and has direct cytotoxic activity. There have been a large number of phase II studies showing the safety and apparent efficacy of this approach.12,13 The most commonly used agent is cisplatin, often combined with other drugs such as fluorouracil (5FU) and gemcitabine. In addition to which drugs are best to use, there are also questions concerning optimal timing of drug delivery in relation to radiation treatment, as well as the potential for induction chemotherapy. Most of the published data also points to the importance of maximal TUR of tumor before consideration is given to any form of bladder-sparing treatment. There is currently a large phase III trial recruiting in the United Kingdom using both 5FU and mitomycin C in combination with external beam radiation therapy. Despite improvements in local control, about 50% of patients with muscle-invasive disease will eventually die with widespread metastatic disease. Untreated, the mean survival of patients with metastatic disease is three to six months.14 With modern multiagent chemo-therapy this figure rises to about one year.14 It is now well-established that multiagent, cisplatin-based regimens offer the best chance of response and survival benefit.15 Until recently the combination of methotrexate, vinblastine, adriamycin (doxorubicin) and cisplatin (MVAC) was the gold standard. A randomized phase III trial was published in 2000 which examined the combination of cisplatin and gemcitabine against MVAC. This trial showed similar response rates (49% vs. 46%), but with significantly less toxicity in those treated with cisplatin/gemcitabine.16 The combination of cisplatin, gemcitabine, and paclitaxel is now being investigated against the new gold standard of cisplatin/gemcitabine in an attempt to further improve results. Summary points: • Intravesical chemotherapy can prevent recurrence of superficial bladder cancer but does not affect progression-free or overall survival • Localized, muscle-invasive bladder cancer can be treated by either primary radical cystectomy or radical radiotherapy with cystectomy reserved for radiation failures • Multimodality therapy is emerging as a useful option with maximal transurethral resection of bladder tumor (TURBT) followed by concomitant chemotherapy/radiation • Metastatic bladder cancer has a poor prognosis, with a median survival of 12 months with modern multiagent chemotherapy • Newer regimens, e. g. cisplatin/gemcitabine, are less toxic but equally efficacious The incidence of prostate cancer is rising and it is now the second most commonly diagnosed cancer in men. It has been recognized for some time that many men diagnosed as suffering from prostate cancer will die of some unrelated cause.17 In view of this and also the potential toxicity of treatment, some patients are advised to avoid specific therapy. Typically this applies to patients with poor life expectancy and with low-grade, good-prognosis tumors, especially if asymptomatic. Fig. 13.2 a Saggital ultrasound scan of the left lobe of the prostate showing a very hypoechoic peripheral zone lesion (arrows) consistent with prostatic carcinoma. b A similar saggital scan in the same patient of the normal right lobe for comparison. The peripheral zone is uniform and echogenic (arrows) Fig. 13.3 Axial T2-weighted scan of the prostate showing extensive low signal change (arrow) throughout the right peripheral zone of the gland. The appearances are those of carcinoma. Tumor extension is noted into the right neurovascular bundle (thick arrow) For purposes of description, the treatment of prostate cancer can most conveniently be divided into: • Treatment of intracapsular disease (Fig. 13.2) • Treatment of localized disease extending outside the capsule, often referred to as locally advanced disease (Fig. 13.3) • Treatment of metastatic disease (Figs. 13.4,13.5) Fig. 13.4 A radioisotope bone scan showing multiple “hot spots” in the spine, pelvis, and one of the ribs on the left. The features are those of metastatic disease. The patient was known to have a prostatic primary. Left-sided hydronephrosis is also noted and the patient is catheterized Fig. 13.5 Coronal T1-weighted MRI scan performed as part of a prostate tumor staging scan. This shows multiple, focal, low-signal lesions throughout both femora, pelvis, and lumbar spine consistent with metastatic disease This is an area of considerable controversy. The main options of active surveillance, radical prostatectomy, external beam radiation therapy, and interstitial therapy have never been compared in a prospective manner. One study has shown no evidence of survival benefit for active therapy over an age-matched control.18 Radical prostatectomy can be performed by the retropubic, perineal, or laparoscopic route with similar results.19 Pelvic lymphadenectomy is performed initially and if frozen section analysis shows tumor involvement, further surgery is abandoned. Nerve-sparing radical prostatectomy is said to result in improved rates of urinary incontinence and impotence. The best results in terms of operative mortality and morbidity come from single-institution centers, but the operation is still regarded as having considerable long-term morbidity; the mortality is 1–1.5%. Total urinary incontinence is rare, but some control difficulties are reported in up to 30% of cases.20 The risk of impotence after radical pros-tatectomy is variously reported as between 24 and 89%. This risk is increased with age and poor pretreatment function. In addition to impotence, attention is also now being paid to overall quality of sexual function after radical prostate cancer treatment but there is little data at present. An alternative treatment for patients with intracapsular disease is a radioisotope implant procedure. Prostate brachytherapy involves the introduction of radioactive isotopes directly into the gland. This is usually performed with the aid of transrectal ultrasound (TRUS) guidance and the radioisotope is delivered through the perineum via a template grid system. Iodine125 and palladium103 are the most commonly-used isotopes. The side effects are similar to external beam therapy, but there is usually slightly greater urinary irritation. The use of prostate brachytherapy has increased dramatically in the last 10 years in tandem with improvements in technology. The main advantage of this form of therapy is its convenience. The procedure involves a maximum of two hospital visits of relatively short duration. The efficacy and toxicity compare favorably to external beam therapy and radical prostatectomy.21 External beam radiation therapy can be used as monotherapy in the treatment of good-prognosis intracapsular disease. Megavoltage beams are used to cover the prostate with a small margin and treatment is usually given daily to a total dose of 64–78 Gy. Escalating radiation dose has been shown to be an independent factor in improving local control and decreasing prostate-specific antigen (PSA) failure, especially in higher-risk patients.22 There have been a number of technological developments over the last 10 years or so which have resulted in improvements in the ability to plan and deliver high doses of radiation to the prostate gland in a consistent and reproducible manner. These include three-dimensional (3-D) treatment-planning systems and computer-controlled delivery systems allowing conformal shaping of radiation beams. It is now possible to vary the beam intensity or fluence of each portal and by so doing the radiation dose distribution can be shaped accurately to the contours of the prostate. This is known as intensity-modulated radiation therapy (IMRT). By using such techniques, doses of up to 81 Gy can be delivered without excessive toxicity.23 Patients with good-prognosis intracapsular disease are given treatment options such as radical prostatectomy, radical external beam radiotherapy, and implantation. A recently published retrospective trial by D’Amico et al.24 examined the PSA failure rate in patients treated by external beam (866 patients), radical prostatectomy (888 patients), or implantation (218 patients). There was no difference in outcome for low-risk patients defined as PSA < 10 ng/mL, stage T2a or less, and Gleason score 6 or less. Whilst some patients with good-prognosis, early-stage disease can undoubtedly be cured by high doses of carefully fractionated external beam radiation, patients who have extracapsular disease, i. e. T3/T4 disease, PSA > 20, Gleason score 8–10, and/or pelvic lymph node involvement, are likely to fail radiation therapy alone. For such high-risk patients the combination of androgen ablation and radiation therapy is often prescribed. Several trials have shown benefit in these situations with regard to biochemical and local control.25,26
Treatment of Bladder Cancer
Treatment of Superficial Disease
Intravesical Therapy
Prognosis
Treatment of Muscle-Invasive Disease
Combined Modality Therapy
Concomitant Chemotherapy/Radiation
Metastatic Disease
Treatment of Prostate Cancer
Treatment of Intracapsular Disease
Treatment of Locally Advanced Disease
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree