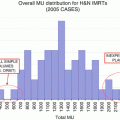
Number
F/U
Age
Tonsil
BT
CS IV
T4
N2b
Dose PTV1
Dose PTV2
Dose PTV3
CRT (%)
SIB
OS
LCR
PFS
Sher
163
36 M
56.1
50
45
75
7
54
70
64
60
100
Yes
86@5Y
86@5Y
Garden
774
54 M
55
48
46
74
17
58
66–72
54
84@5Y
90@5Y
82@5Y
Setton
442
36.8 M
57
50
46
73
13.8
69.5@N2–3
70
59.4
54
95
Yes
84.9@3Y
LR5.4 %@3Y
Daly
107
29 M
70 %@60>
44
48
85
29
83@N2-3
66
54
50
98
Yes
83.3@3Y
92@3Y
81@3Y
Huang
71
33 M
55
38
61
76
11
72@N2-3
70
59.4
54
100
Yes
83.3@3Y
90@3Y
81@3Y
Clavel
100
35 M
46
58
39
87
12
87@N2–3
70
59.4
50.4
100
Yes
92.1@3Y
95.1@3Y
85@3Y
In this chapter, we have introduced practical guidance and evidence supporting the use of IMRT to treat patients with OPC.
9.2 Epidemiology
The oropharynx comprises the soft palate (SP), uvula, tonsillar fossa (TF) and pillars, glossotonsillar sulci, lateral and posterior pharyngeal wall, vallecula, and base of the tongue (BOT). It is located between the soft palate (superior) and hyoid bone (inferior). The oral cavity is located anterior to the oropharynx. These subsites have been divided into four sections: the anterior wall (AW; BOT, vallecula), lateral wall (LW; TF, pillars, glossotonsillar sulci, lateral pharyngeal wall), superior wall (SW; inferior surface of SP, uvula), and posterior wall (PW; posterior pharyngeal wall). Approximately 60 % of OPC consists of the LW, followed by the AW, SW, and PW.
There is a rich lymphatic network, in which early lymphatic involvement typically develops, with approximately 60 % of diseases being diagnosed as stages III–IVB. The most common histopathology of OPC is squamous cell carcinoma (SCC). Poorly differentiated histopathology is frequently reported in the TF and BOT, while more differentiated histological subtypes have been observed in the other sites. Apart from SCC, histopathological findings have revealed adenocarcinoma, adenoid cystic carcinoma, mucoepidermoid carcinoma, and malignant lymphoma.
A well-known risk factor for OPC is smoking, alcohol consumption, diet, poor oral hygiene, marijuana consumption, and clinical stage. Human papillomavirus (HPV) is becoming more frequently associated with OPC, and incidence has been reported in more than half of HNC patients [1]. HPV is most commonly found in the TF and BOT, with HPV16 being identified as the main population. HPV status has been shown to be a distinct risk factor. Ang et al. proposed a risk model of OPC using recursive-partitioning analysis (RPA) on the basis of four factors [5]: HPV status, pack years of tobacco smoking, tumor stage, and nodal stage. Of 743 patients from the RTOG 0129, who were randomly assigned to receive accelerated fractionation RT with cisplatin (CP) or standard fractionation RT with CP, there were 433 patients (60.1 %) with OPC [24]. HPV status information was obtained from 322 patients in these groups, and they were entered into this analysis. HPV-positive patients (n = 206) had significantly better overall survival rates (OS, 82.4 % vs. 57.1 %; p < 0.001) than those of HPV-negative patients. They were further categorized into three groups based on the OS with RPA, and the 3-year OS rates in low-risk (n = 114), intermediate-risk (n = 79), and high-risk groups (n = 73) were 93 %, 70.8 %, and 46.2 %, respectively. Therefore, HPV status will be regarded as more essential information in future clinical practices [5–10] (Table 9.2).
Table 9.2
Correlation of HPV status with overall survival in reported series of radiotherapy for oropharyngeal cancer
Patient number | HPV positive | HPV negative | |
|---|---|---|---|
Ang | 223 | 84 %@3Y | 51 %@3Y |
Rischin | 185 | 91 %@2Y | 74 %@2Y |
Shi | 111 | 88 %@3Y | 67 %@3Y |
Lassen | 74 | 66 %@5Y | 28 %@5Y |
Fakhry | 62 | 84 %@3Y | 50 %@3Y |
Nichols | 44 | 89@3Y | 65@3Y |
9.3 Clinical Work-Up
Clinical presentation, patient history, and physical examination are considered essential procedures for a diagnostic work-up. A comprehensive evaluation of the head and neck including the oropharynx, oral cavity, nasopharynx, hypopharynx, and larynx is necessary, and biopsy is a confirmative method used for a diagnostic assessment of the extent of the tumor [25]. Panendoscopy is routinely recommended to detect second primary malignancy [26], and, if possible, narrowband imaging techniques are helpful for finding superficial lesions in both the esophageal and head and neck regions [27]. Early SP tumors sometimes invade superficially with erythroplasia; therefore, careful estimations of the entire mucosa and pathological confirmation should be considered, where necessary.
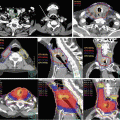
CT with contrast media is the standard diagnostic imaging technique, and magnetic resonance imaging (MRI) is also recommended to accurately determine both primary tumor extension and normal tissue boundaries with sufficient spatial resolution of the soft tissues. BOT tumors are typically bulky and expand to the vallecula, TF, and mobile tongue; therefore, MRI should be mandatory because of the valuable information obtained regarding the extent of the disease. MRI is also advantageous because it is less sensitive to dental artifacts than CT [28] (Fig. 9.1). Neck lymph nodes are considered to be positive if the smallest diameter measured is greater than 1 cm. Determining the presence or absence of extracapsular invasion is of importance; therefore, thin slice CT or MRI assists in an accurate evaluation. 18Fluorodeoxyglucose (FDG) positron-emission topography (PET) exhibits high accuracy for tumor involvement [29] and can be used to screen for distant metastasis [30]. FDG-PET scans may be recommended especially in the case of advanced disease. FDG-PET scans also have the ability to accurately assess the treatment response of patients treated with RT and/or chemotherapy [31]. The recommended timing of FDG-PET after RT is 2–3 months, because an earlier assessment has been shown to increase the false-positive rate due to acute radiation reactions.


Fig. 9.1
CT image (a) and T2-weighted MRI image (b) of patients with tonsillar carcinoma (T2N0M0). A dental filling artifact on the CT image (arrow head) greatly interfered with the detection of the primary tumor, while it can be clearly detected on the MRI image due to reduced sensitivity to metallic artifacts
Integrated PET/CT imaging has provided interactive information on both anatomical and functional volumes. FDG-PET data can be directly imported to an RT treatment planning workstation, at which researchers reported that integrated PET/CT planning was advantageous for clinical outcomes [32].
Target volume delineation is crucial in the setting of IMRT; therefore, diagnostic information has become more important to refine the quality of treatments used.
A consultation with a dentist regarding management of the disease prior to starting RT is essential, because extraction after RT leads to increase the incidence of critical adverse events, such as osteonecrosis and trismus. Education on how to stop smoking before RT is initiated is also very important because smoking has been shown to decrease the efficacy of RT and increase the risk of both adverse events and secondary cancer.
9.4 Clinical Indication
Since the treatment of OPC is complex, a multidisciplinary team board should ideally determine clinical decisions. The tumor-node-metastasis classification (TNM) stage, diagnostic imaging, pathological findings, patient condition, and social background are essential when determining an adequate treatment modality for OPC (Fig. 9.2).


Fig. 9.2
Overview of clinical strategy regarding radiotherapy for patients with oropharyngeal cancer according to clinical stage and practical background
#1 Disease with T2N1 of the tonsil or base of the tongue is candidate for radiotherapy alone
#2 Concurrent use of cytotoxic agents is not supported by evidence
#3 Platinum component (level IA) and cetuximab (level Ib) are considered to be administered
#4 Combination use of taxane, 5-FU, and cisplatin is preferred
#5 Chemoradiotherapy is considered for high-risk features such as positive margin and/or extracapsular invasion
The mainstay of treatment for OPC is both surgery and RT. External beam RT is typically administered in 70 Gy/35 fractions over 7 weeks with a standard fractionation schedule. A meta-analysis from 15 trials with 6,515 patients revealed that an altered fractionation schedule had significant advantages for local control and OS [33] (level Ia). The majority of cohorts were comprised of OPC patients (47.2 %) and stage III patients, which were categorized as an intermediate-risk group, and these groups had slightly better OS in subset analysis. One of the reasons for this patient population is that physicians consider CRT to be a better strategy for patients with advanced nodal disease because of the higher risk of distant metastasis. If altered fractionation is selected, the limitations associated with the combination use of chemotherapy should be considered (which is described later). Brachytherapy [34] and stereotactic radiosurgery [35] are considered better options for minimizing the radiation dose to normal organs such as the parotid gland, mandibula, and pharyngeal constrictor muscle.
A trans-oral approach or endoscopic mucosal resection is considered an adequate option for localized small primary lesions without nodal spread because sufficient outcomes for efficacy and function can be achieved [36]. Postoperative RT should be added if a positive surgical margin is revealed in the pathological specimen; however, this has been associated with a higher risk of adverse events than single modality treatment [37–39]. RT is believed to be more advantageous for both organ and functional preservation than open surgery. IMRT is strongly recommended to minimize the late adverse events associated with RT, especially xerostomia [40, 41].
Patients with moderately advanced disease are also considered as good candidates for definitive RT. Patients with larger tumors (T2–T3) and/or nodal involvement (N1–N2) typically received RT with chemotherapy or molecular-targeted agents. The concurrent use of platinum-based components was distinctly beneficial for OS and disease control [42, 43] (level Ia), and it is considered a standard treatment for locally advanced disease. In the case of concurrent chemoradiotherapy (CCRT), 70 Gy/35 fractions over 7 weeks is the standard RT schedule. The RTOG 0129 trial [24], which enrolled 743 patients with stage III–IV disease, reported that accelerated fractionated (AX) RT with CP did not have any advantage over standard fractionated RT with CP [24]. Therefore, AX combined with CCRT should be used in clinical trial settings.
One randomized trial, RT with cetuximab (CET; anti-epidermal growth factor receptor (EGFR)), showed improvements in OS and local control over those with RT alone [44, 45]. In the Bonner trial, 424 patients with stage III–IV OPC, hypopharynx, and larynx cancer were randomly assigned to the RT with CET arm or the RT arm. The RT with CET arm showed significant improvements in locoregional control (HR = 0.68, p = 0.005) and OS (HR = 0.74, p = 0.03). Mortality was lower in the RT with CET arm than in the RT arm without an increase in the incidence of adverse events. In this trial, the RT schedule included three types of fractionation schedules without stratification at randomization, such as standard fractionation, hyperfractionation, and the concomitant boost method (AX). Subset analysis revealed better results with the concomitant boost method than the other schedules. We could not obtain the prospective trial results for the direct comparison between CP and CET until the end of 2013. The RTOG 1016, a phase III trial of RT plus CET versus CCRT with CP in HPV-associated OPC, is now ongoing. Results will be obtained from this trial in the near future (RTOG 1016). CET was added to CCRT to assess improvements in the clinical results of CCRT without increasing the toxicity. The RTOG 0522 trial was designed to compare the CCRT with CET arm to the CCRT arm [46]. The findings of this trial failed to show the clear advantages of adding CET to CCRT; however, it revealed the increased incidence of acute adverse events in the combined arm. Based on these findings, CCRT with anti-EGFR should be tested in clinical trials, and care should be taken for its clinical use.
Adequate timing for the addition of RT to chemotherapy is believed to be concurrent administration [43]. When induction and adjuvant setting were compared, CCRT caused the largest benefits in OS and locoregional control associated with increasing acute toxicity. A meta-analysis showed that induction chemotherapy (IC) was moderately beneficial for OS and was more advantageous for the control of distant metastasis [43]. IC with taxane-containing multi-agents showed apparent advantages for OS and disease control than those of CP and 5-FU in both randomized control studies [47, 48] and meta-analysis [49]. According to the results from meta-analysis, the addition of taxane components to CP and 5-FU was distinctly beneficial for both OS (HR 0.79 95 % CI 0.7–0.89) and disease control (HR 0.78 95 % CI = 0.69–0.87; level Ia). Highly advanced disease (T4 and/or N3 disease) is associated with a higher risk of distant metastasis; therefore, this is a reasonable strategy for these groups. However, intensive IC may lead to low CP compliance (only 50 %) in subsequent CCRT phases [49]. Another clinical utility of IC is as a strategy for organ preservation [50–53]. If a sufficient response is acquired from IC, patients with sufficient responses could take the clinical advantage of selecting RT over up-front surgery. Patients without a sufficient response have to undergo surgery and be subjected to the high-risk features associated with postoperative radiotherapy [37, 38] (see details in Chap. 11).
Since either primary or lymph node bulky lesion may greatly decrease the efficacy of RT, surgical approaches may be necessary for disease control. Functional loss from surgery should also be taken into careful consideration in such cases. A multidisciplinary team board is needed to coordinate both treatment efficacy and morbidity.
Definitive RT (with chemotherapy) is the standard treatment for unresectable disease, such as stage IVB disease. If tumors present with rapid growth and/or a very large volume, the benefits associated with IMRT may be decreased because of both the long preparation time and decreased ability to spare the dose delivered to the normal tissue due to a large CTV. 3D conformal RT may be a more practical application for such cases.
The mainstay of treatment for stage IVC disease is systemic chemotherapy, and RT is chiefly considered as a palliative intent for symptom relief. If patients have significant hazards due to airway obstructions, uncontrollable bleeding, and refractory cancer pain, CCRT still has a sufficient impact on locoregional control for the selected patients with sufficient organ function.
9.5 Target Delineation and Dose Prescription
IMRT can uniquely be administered at variable dose levels to different clinical target volumes (CTVs). The simultaneous integrated boost (SIB) technique is generally used in IMRT for HNC. CTVs for primary lesion and involved nodes are generally delivered to approximately 70 Gy over 6–7 weeks and other CTVs with risk level treated with 54–63 Gy with the same treatment duration. The advantage of the SIB technique is that it is simple and convenient for practical management. The clinical benefits of adaptive replanning have recently been reported in several studies [54–56] (see Chap. 7). IMRT can be administered at steep dose gradients between CTV and normal tissue; therefore, anatomical changes due to tumor shrinkage and/or body weight loss lead to significant differences in the dose delivered. Adaptive replanning may resolve the problem of unexpected dose changes during the treatment course; however, the effort associated with extra-planning procedures would increase. Another problem linked to the SIB technique is that CTVs of risk level are treated with fraction sizes smaller than 1.8 Gy, which may decrease the efficacy of its biological radiation effects. The two-step plan, which is similar to conformal RT with the cone-down technique, could extrapolate experiences from standard fractionation schedule [54]. Advances in both the treatment planning apparatus and sophisticated integrated imaging system may warrant exploring more convenient achievements in adaptive replanning.
9.5.1 Gross Tumor Volume (GTV)
The anatomy of the oropharynx is very complex; therefore, integrating all clinical information from physical examinations, such as inspection and palpation, and diagnostic imaging from CT, MRI, fiber-optic images, and PET scans is of importance. These diagnostic images should be taken in identical head positions to those taken during the CT simulation with an immobilization mask. If possible, a metallic marker should be placed at the landmark of the primary tumor to achieve accurate delineation. In addition, the volumetric 3D reconstruction of GTVs in coronal and sagittal views on the treatment planning system is helpful for assessing the extension of the primary lesion. Regarding the lymph node evaluation, if its shortest diameter is equal to or greater than 1 cm, it is defined as lymphatic involvement. In addition, the clinical features of the lymph nodes with peripheral rim enhancement or positive PET finding are also considered to be the feature of lymphatic involvement. Clinical features of borderline findings with either primary lesion or lymph node involvement are regarded as GTVintermediate-risk. GTVintermediate–risk typically expands with a 1-cm margin surrounding GTVprimary. If patients receive neoadjuvant chemotherapy prior to IMRT, GTV should be carefully determined according to initial diagnostic findings and not those taken after chemotherapy. Insufficient contouring toward microscopic tumor deposits modified by the chemotherapy response may decrease curability by RT, especially in the case of IMRT.
9.5.2 Clinical Target Volume (CTV)
CTV is typically arranged using automated volumetric expansion on a treatment planning system and encompasses GTVprimary, GTVnode, and GTVintermediate-risk areas. The CTVprimary margin is generally defined as 10 mm in three dimensions, minus the anatomical boundaries without invasion. CTVnode margin typically was defined as 5-mm surrounding GTVnode, while the extent of the margins should be increased according to adverse features such as extracapsular invasion. Whether normal structures adjacent to GTV are involved or not, both the anatomical location and biological features of the primary tumor should be carefully considered for the delineation of CTV. Clinical encounters and integrated information by radiation oncologists are crucial for deciding the CTV boundary and greatly correlate with performance in IMRT. Thus, expert radiation oncologists must carefully edit automatically made CTV. Both CTVprimary and CTVnode were generally treated with a dose of approximately 70 Gy. Using SIB technique, doses of 60–63 Gy are commonly delivered to CTVintermediate-risk.
CTV for prophylactic nodal areas is commonly defined independently. The lymph node level varies with the T and N stages and the distribution of nodal involvement [57] (Table 9.3). Bilateral levels II to V and retropharyngeal node area will typically be included for locally advanced carcinoma. Level Ib area may also be covered in case of positive nodes in level II and/or oral cavity invasion. If lower neck node involvement is observed, the supraclavicular lymph node would be covered in the prophylactic nodal area. The level V area can be excluded from the prophylactic nodal area in the case of N0 disease. Unilateral nodal CTV may be considered in early and intermediate-risk patients with favorable outcomes, such as those with localized TF lesions with T1–2 N0–1 [58].
Table 9.3
Incidence (%) of lymph node involvement according to subsites and T stage in patients with oropharyngeal cancer
T stage | N0 | N1 | N2 | |
|---|---|---|---|---|
Oropharyngeal wall | T1 | 75 | 0 | 25 |
T2 | 70 | 10 | 20 | |
T3 | 33 | 23 | 45 | |
T4 | 24 | 24 | 52 | |
Soft palate | T1 | 92 | 0 | 8 |
T2 | 64 | 12 | 25 | |
T3 | 35 | 26 | 39 | |
T4 | 33 | 11 | 56 | |
Tonsillar fossa | T1 | 30 | 41 | 30 |
T2 | 33 | 14 | 54 | |
T3 | 30 | 18 | 52 | |
T4 | 11 | 13 | 77 | |
Base of the tongue | T1 | 30 | 15 | 55 |
T2 | 29 | 15 | 57 | |
T3 | 26 | 23 | 52 | |
T4 | 16 | 9 | 76 |
9.5.3 Planning Treatment Volume (PTV)
PTVs are defined as CTVs with adequate margin providing sufficient dose coverage and a setup error. The range of the PTV margin may vary depending on the features of the IMRT system used in each institute. Details have been described in Chap. 5.
9.5.4 Organ at Risk (OAR) and Planning Organs at Risk Volume (PRV)
Ten or more OARs are typically prepared for treatment planning. The spinal cord, brainstem, parotid gland, mandibula, inner ear, optic pathway, eye and lens, oral cavity, pharyngeal constrictor muscle, and larynx are commonly used for IMRT planning. If needed, the submandibular gland, thyroid gland, brachial plexus, masticator muscle, and temporomandibular joint can also be used. Special care should be taken for dose sparing for submandibular gland, especially in cases of ipsilateral level II involvement. Dose sparing toward submandibular gland may increase the risk of an insufficient dose being delivered to neighboring PTV in such cases (Fig. 9.3). As for critical organs such as the spinal cord and brainstem, PRV with expanding 5 mm to the OAR is commonly added (see details in Chap. 5). Dummy objects that improve the conformal dose distribution in IMRT are typically used and depend on the institutional IMRT system including the treatment machine. For example, a dummy object on the back of the neck is commonly used to reduce the dose delivered to the soft tissue in the posterior neck region.