Fig. 1
Direct local tumor extension from adjacent sites (the fallopian tube, uterus, urinary bladder, colon) to the ovaries. Green arrows indicate infiltrated visceral lymph nodes surrounding the primary tumor
Spread from distant sites occurs mainly via blood and lymph vessels and/or through transcoelomic dissemination with surface implantation (Fig. 2). The fallopian tube presents yet another route for possible metastatic spread from a carcinoma of the uterine corpus or cervix or the fallopian tube itself onto the ovarian surface (Fig. 2). Three main categories are suggested for the classification of site-specific tumors: (1) spread from extragenital sites, (2) spread from other sites in the genital tract, and (3) involvement by peritoneal tumors.
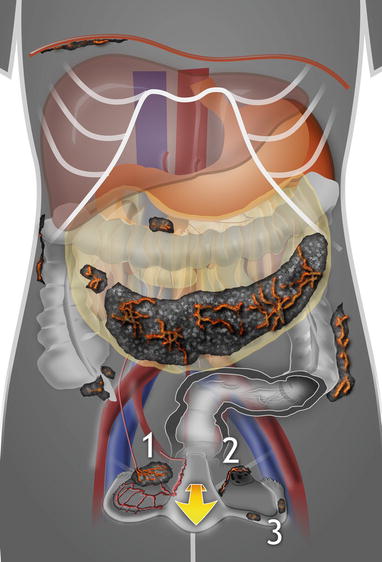

Fig. 2
Routes of tumor spread from distant extragenital sites. 1 Hematogenous and lymphatic spread, 2 generalized peritoneal spread of extraovarian primary tumor with ovarian surface implantation (visceral carcinomatosis); in this type of spread, foci of metastatic carcinoma located on the ovarian surface or superficially within the ovarian cortex are encountered, 3 spread of genital tract carcinomas (endocervical, endometrial, or tubal tumors) through the lumen of the fallopian tube onto the ovarian surface. Findings of highly perfused solid bilateral tumor, or tumor on the surface of the ovary without significant involvement of the underlying parenchyma, are suggestive of metastatic origin
A metastasis to the ovary must be strongly considered if the distribution of disease or tumor sonomorphology is atypical for primary ovarian cancer. In such cases, a minimally invasive biopsy should be performed prior to primary cytoreductive surgery for ovarian malignancy. The optimal technique to achieve an adequate histological sample in one setting is ultrasound-guided tru-cut biopsy [2, 3]. As with other bioptic techniques, this procedure should be avoided in cases of severe thrombocytopenia and other serious coagulative disorders, when the tumor is in a risky location or when the tumor capsule remains intact with no sign of spread. If all requirements can be met and the histological sample is obtained, it should be then subjected to thorough examination by an experienced pathologist using additional sections, special stains, and immunohistochemistry with the goal to pinpoint the origin of the primary tumor. Needless to say, this is of importance not only regarding the possible difference in treatment but also regarding the location of the primary tumor that in such cases is often not yet known. If the ovarian tumor is confirmed as metastatic and its histological origin ascertained, then the subsequent search for an extraovarian primary tumor could be sufficiently targeted, thus avoiding unnecessary laparotomies and allowing for timely initiation of an appropriate treatment. In some cases, the primary intra-abdominal tumor is discovered on ultrasound together with the metastatic ovarian tumor, or ovarian tumor suspect of metastatic nature is found during a patient follow-up after treatment for a non-ovarian tumor. In such situations, it is of benefit to compare the primary tumor histotype with an ovarian biopsy to exclude eventual concurrent primary ovarian neoplasm.
General Principles
Among the general principles that aid the sonographer in arriving at correct diagnosis of a metastatic tumor are (1) thorough clinical history aimed at the existence of concurrent or prior tumor in another organ, (2) awareness of the frequency with which the suspected primary tumor metastasizes and of its routes of spread, (3) targeted questioning about clinical symptoms attributable to primary origin of neoplasia, (4) evaluation of blood tests and tumor markers if available, (5) systematic performance of transvaginal and transabdominal ultrasound, and (6) knowledge of sonomorphologic and Doppler patterns of the most common ovarian metastatic tumors, e.g., (A) the presence of ovarian bilateral lesions, (B) the findings of multiple tumor nodules (nests) within the ovary or (C) tumor on the surface of the ovary without significant involvement of the underlying parenchyma, (D) absence of a purely unilocular or multilocular ovarian tumor pattern, and (E) high color score in solid portion of ovarian masses on Doppler. However, many exceptions exist; for example, serous and undifferentiated primary ovarian carcinomas are commonly bilateral and may present with multinodular appearance involving ovarian parenchyma and/or with ovarian surface location.
Accurate diagnosis of the nature and origin of ovarian metastatic tumors is only possible if detailed transvaginal ultrasound is combined with transabdominal scan. Such a systematically performed ultrasound examination allows the detection of primary extraovarian tumor and/or concurrent disseminated disease (i.e., liver parenchymal metastases, visceral lymph nodes surrounding the primary tumor, carcinomatosis incl. omental infiltration, and ascites) [4]. The gray-scale ultrasound is combined with color or power Doppler evaluation of vessel density and their architecture. The diagnosis is usually made based on two-dimensional (2D) scan. Any additional value of three-dimensional ultrasound in diagnosing this population of tumors has yet to be ascertained. A subjective semiquantitative assessment of the amount of detectable blood flow within the tumor is made by using a color score (1, no color; 2, minimal color; 3, moderate amount of color; 4, abundant color density detected in the tumor) [5]. Using subjective color Doppler score [5], these masses are usually highly vascularized and, in a study by Guerriero et al. [6], 50–82 % of them presented with color score 4 (abundant perfusion). The dynamic 2D color or power Doppler also enables visualization of newly formed vessels entering the tumor from surrounding structures. Testa et al. observed that cases of metastases to the ovaries are characterized by a main peripheral vessel which penetrates into the central part of the ovarian mass with a tree-shaped morphology [7]. In addition, in metastatic ovarian masses presenting with multinodular appearance, we typically observe ring-shaped vessels around the nodules that may highlight their presence (Fischerova D. 2012, unpublished data).
Currently, there are no known tumor markers for specific types of cancer; however, general serum tumor markers are often elevated in disseminated disease and are being used clinically for monitoring the course of disease and relapse detection. Therefore, the evaluation of one or more tumor markers may contribute to the diagnosis of metastatic ovarian tumor and the detection of its primary source. Most serum tumor markers are tumor antigens (carcinoembryonic antigen [CEA], CA 19–9, CA 72–4, CA 15–3, CA 27.29, CA-125). Elevated CEA levels are found in a variety of cancers including colonic, pancreatobiliary, gastric, lung, breast, endocervical, and ovarian [8, 9]. They are, however, also often detected in noncancerous conditions that include cirrhosis, inflammatory bowel disease, chronic lung disease, and pancreatitis. Normal CEA level in an adult nonsmoker is <2.5 ng/mL and in a smoker <5.0 ng/mL. CA 19–9 (normal level <37 U/mL) is usually greatly elevated in advanced pancreatic carcinoma (in 71–93 % cases) and in about 65 % cases of bile duct cancer [9]. Elevated levels are also seen in 21–42 % cases of gastric cancer, 20–40 % cases of colon cancer, and in nonmalignant conditions such as chronic pancreatitis, cirrhosis, and acute cholangitis [10] as well as in cases of noncancerous bile duct obstruction. CA 72–4 is elevated in 40 % cases of gastrointestinal and 50 % cases of ovarian cancer [11]. CA 15–3 and CA 27.29 can detect advanced breast cancer and its recurrence after primary treatment [12, 13]. The normal range of CA 15–3 and CA 27.29 is <31 U/mL and <40 U/mL, respectively. In squamous cell cervical cancer, the serum concentrations of squamous cell carcinoma antigen (SCC) have been found to correlate with tumor stage, tumor size, residual tumor after treatment, recurrent or progressive disease, and patient survival [14]. Its normal level is <2.5 ng/mL. The serum CA-125 (cutoff value 35 U/mL) is elevated in nonmucinous ovarian carcinoma but also in endometrial, pancreatobiliary, lung, breast, and colon cancer and in noncancerous conditions including endometriosis, pelvic inflammatory disease, uterine myomas, and cirrhosis [9, 15]. The serum CA-125 level may be also elevated in secondary ovarian neoplasms contributing to potential confusion with primary ovarian neoplasia. Recent study by Calster et al. presented likelihood reference tables to interpret preoperative serum CA-125 results in patients with different ovarian pathologies [16]. In metastatic ovarian tumors, the median concentration of serum CA-125 in all population and in premenopausal and postmenopausal patients reached 99, 80, and 132 U/mL, respectively. Metastatic tumors were associated with CA-125 levels similar to stage I primary invasive tumors but with lower levels than higher stage primary invasive tumors (median: 229 U/mL for stage II, 401 U/mL for stage III, and 725 U/mL for stage IV) and higher stage borderline tumors (median: 165 U/mL for stage II and 327 U/mL for stage III–IV). Other useful tumor markers are chromogranin A (CgA) associated with neuroendocrine tumors and beta2-microglobulin (B2M) and lactate dehydrogenase (LDH) associated with lymphomas. A urine marker—bladder tumor antigen (BTA)—is frequently detected in cases of urothelial cancer. In addition, excessive production of estrogens, androgens, or progesterone does not exclude a metastatic tumor that may have a functioning stroma [17].
This combined diagnostic approach (knowledge of patient history and clinical symptoms, blood tests, and systematically performed ultrasound examination) enables the diagnosis of extraovarian metastatic tumors with sensitivity of 84.8 %, specificity of 92 %, positive and negative predictive value of 82.3 and 93.3 %, respectively, and accuracy of 89.9 % [2].
In many cases, when ultrasound examination is highly suggestive of extraovarian primary origin, a minimally invasive biopsy (optimally, an ultrasound-guided tru-cut biopsy) of ovary or other metastatic lesion (omentum, peritoneum, lymph nodes) is performed. If a transabdominal scan or other complementary imaging techniques reveal the potential site of primary tumor (stomach, pancreas, bile duct, colon, etc.), then endoscopy or endosonography permitting the biopsy of primary tumor is added. Once a diagnosis of metastatic disease is established, the patient is referred to a multidisciplinary team for appropriate staging and optimal oncological treatment.
Site-Specific Tumors
The establishment of specific (pathognomonic) ultrasound features that could be reliably used for differential diagnosis between primary and metastatic ovarian neoplasms presents a great challenge. Very few studies were performed with the aim to identify possible specific ultrasound characteristics of metastatic ovarian malignancies [6, 7, 18–21]. So far, none of the features initially promising in terms of assisting in diagnosis (e.g., the presence of lead vessel, necrosis, papillary projection specifically described in intestinal metastases to the ovary, mobility or elasticity of tumor, tumor heterogeneity) were prospectively validated. In addition, data revealing the tumor laterality might be compromised as in some cases the laterality of ovarian metastasis was diagnosed on ultrasound first and only suspect ovarian tumors were verified by tru-cut biopsy [21] or, in other studies, the sectioning of ovaries necessary for the detection of microscopic foci was not performed in sufficient detail. Moreover, in many studies the ultrasound-evaluated morphology of ovarian metastases was associated only with the original site of primary tumor and not with its histotype, not taking into account that many tumor histotypes with quite different gross appearance may derive from a single organ. For example, ovarian metastases from the appendix may be derived from at least three histological tumor types: (1) intestinal type or mucinous carcinoma presenting within the ovary as multilocular-solid masses, (2) signet ring cell carcinoma (Krukenberg tumor) manifesting in the ovary as a solid mass with a lead vessel, and (3) low-grade mucinous tumors resembling a primary ovarian mucinous intestinal borderline tumor. This implies that, in order to aid in the diagnostic process, the association of ultrasound morphology should be made primarily with the tumor histotype and only secondarily with the presumed site of its origin.
However, useful guidelines may still be derived from multiple observations that could be of help to an experienced sonographer in the process of diagnosing a secondary ovarian neoplasm as well as in identifying its original source. In general, ovarian metastases from signet ring cell tumors (Krukenberg tumors) usually derive from the stomach and rarely from large intestine, appendix, and breast; neuroendocrine tumors (e.g., of small intestine, appendix, colon, stomach, pancreas, or lung), breast carcinoma, and secondary ovarian lymphoma are typically bilateral solid lesions (Fig. 3), whereas ovarian metastases derived from colorectal or pancreatobiliary adenocarcinoma show multilocular-solid morphology (Fig. 4). Metastatic tumors tend to appear moderately or highly vascularized [6]. However, the sonomorphology and perfusion of ovarian metastases may change during the course of treatment.
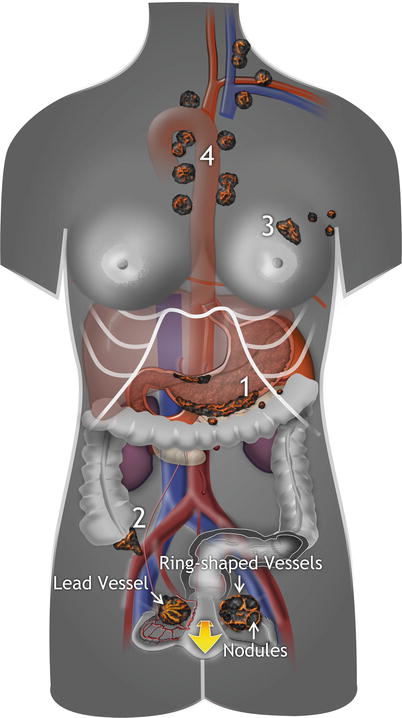
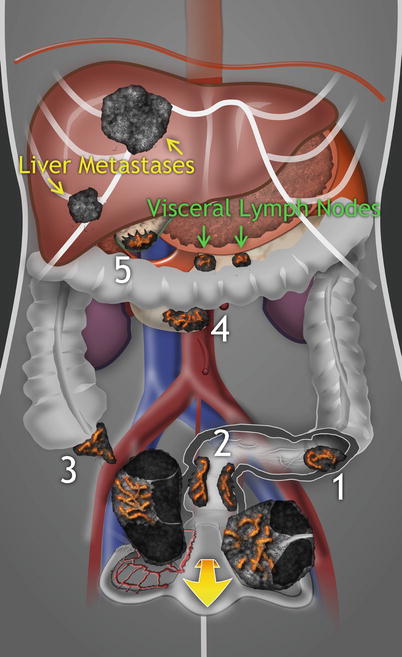

Fig. 3
Examples of primary origin of solid ovarian metastases. 1 Signet ring cell carcinoma (Krukenberg tumor) of gastric pylorus with multiple infiltrated visceral lymph nodes, 2 neuroendocrine tumor of the appendix, 3 breast carcinoma with multiple infiltrated axillary lymph nodes, 4 mediastinal lymphoma. Of note, the presence of multiple tumor nodules with ring-shaped vessel arrangement within the left ovary, and the lead vessel penetrating the right ovary with a tree-shaped architecture, is a finding typical for ovarian metastasis. These metastatic lesions are frequently accompanied by peritoneal involvement and ascites

Fig. 4
Examples of origin of multilocular-solid ovarian metastases. Adenocarcinoma of sigmoid colon (1), rectum (2), appendix (3), pancreas (4), and biliary tract (5), often accompanied by parenchymal liver metastases and infiltrated visceral lymph nodes adjacent to the primary tumor. Of note, the ovarian metastases often have multilocular-solid appearance even when there is absence of cysts in the primary neoplasm
In addition, a combination of detailed tumor sonomorphology with vessel tree arrangement visualized on Doppler may play a role in distinguishing primary and metastatic ovarian tumors of solid appearance. According to our observation, the presence of multiple hypoechogenic nodules located within the ovary (like tumor “nests”) together with ring-shaped vessel arrangement detected on Doppler is often found in solid metastatic ovarian tumors commonly originating from signet ring cell carcinomas or breast cancer and other metastatic carcinomas. The multiple nodules within the ovarian tissue are due to embolic tumor spread, which is commonly accompanied by prominent intravascular tumor nests in the ovarian hilum, mesovarium, and mesosalpinx [22]. Moreover, Testa et al. described in more than 50 % of solid tumors the presence of lead vessel entering the tumor and penetrating the central part of the ovarian mass with a tree-shaped morphology [7].
Differential diagnosis between primary and metastatic ovarian tumors of multilocular-solid appearance in cases when biopsy proves mucinous histotype is often challenging. Using a simple rule suggested by Seidman at al. [23] and modified by Yemelyanova et al. [24] that classifies all bilateral mucinous carcinomas of any size and all unilateral mucinous carcinomas <13 cm as metastatic and unilateral mucinous carcinomas ≥13 cm as primary ovarian tumors, 82 % of ovarian metastases and 98 % of primary ovarian tumors can be correctly classified. Unfortunately, there are many exceptions to this algorithm, especially in cases of colorectal and endocervical carcinomas. Furthermore, the findings of implants on the surface of the ovary and/or multinodular ovarian tumors with mucinous features merit more serious consideration due to the possibility of being metastatic. Similarly, the distribution of disease may be helpful in distinction of primary and metastatic mucinous tumors involving the ovaries. For example, the presence of hepatic metastases and/or extensive peritoneal dissemination is an unusual pattern of spread for a primary ovarian mucinous cancer.
The characteristics of most common extraovarian metastatic tumors involving the ovaries are presented below.
Spread from Extragenital Sites
Carcinoma of the Stomach
Metastatic Tumor with Signet Ring Cells (Krukenberg Tumor)
Krukenberg tumor is characterized by the presence of mucin-filled signet ring cells accounting for at least 10 % of its mass. The source of metastatic Krukenberg tumor in the ovary is in a great majority of reported cases of gastric carcinoma. Carcinomas of the large intestine, appendix, and breast are the next most common primary sites; the gallbladder, biliary tract, pancreas, cervix, and urinary tract are rare sources of these tumors [25]. The average age of patients with Krukenberg tumor is about 45 years [26]. The age distribution is related to higher frequency of signet ring cell carcinomas in younger women and to the greater vascularity of the ovary in premenopausal women. A history of prior carcinoma of the stomach or another organ is present in 20–30 % of the cases, and disease-free interval is usually 6 months or less [27]. Almost all patients die within a year of the diagnosis of ovarian metastasis [28].
Diagnosis
Almost 90 % of patients with Krukenberg tumor have symptoms related to ovarian involvement. The gastrointestinal symptoms are frequently vague with weight loss, epigastric discomfort, nausea, vomiting, loss of appetite, fatigue, and early satiety. Miscellaneous symptoms are related to the spread to other sites such as lungs or bone. Tumor markers CEA and CA 19–9 are elevated in 40–50 % of patients with disseminated disease, and monitoring of these markers may be beneficial in the detection of recurrence [14].
On transvaginal scan, the Krukenberg tumor typically appears as a rounded or reniform richly vascularized solid mass (Fig. 5). Occasionally, these tumors contain cysts filled with anechogenic (watery) or hypoechogenic (mucinous) fluid separated by solid tissue (Fig. 6). Both ovaries are involved in 80 % or more of cases [29]. In differential diagnosis the Krukenberg tumor may resemble any primary solid ovarian tumor, e.g., ovarian fibroma or dysgerminoma. However, unlike the Krukenberg tumor, both these tumors are usually unilateral and of larger size. Fibromas also usually present with stripy shadows and minimal vascularity, while dysgerminomas differ from Krukenberg tumors in age distribution as they are mostly found in young women 20–30 years of age [30, 31]. Another distinguishing feature could be the finding of pelvic and abdominal peritoneal implants characteristic for Krukenberg tumor.
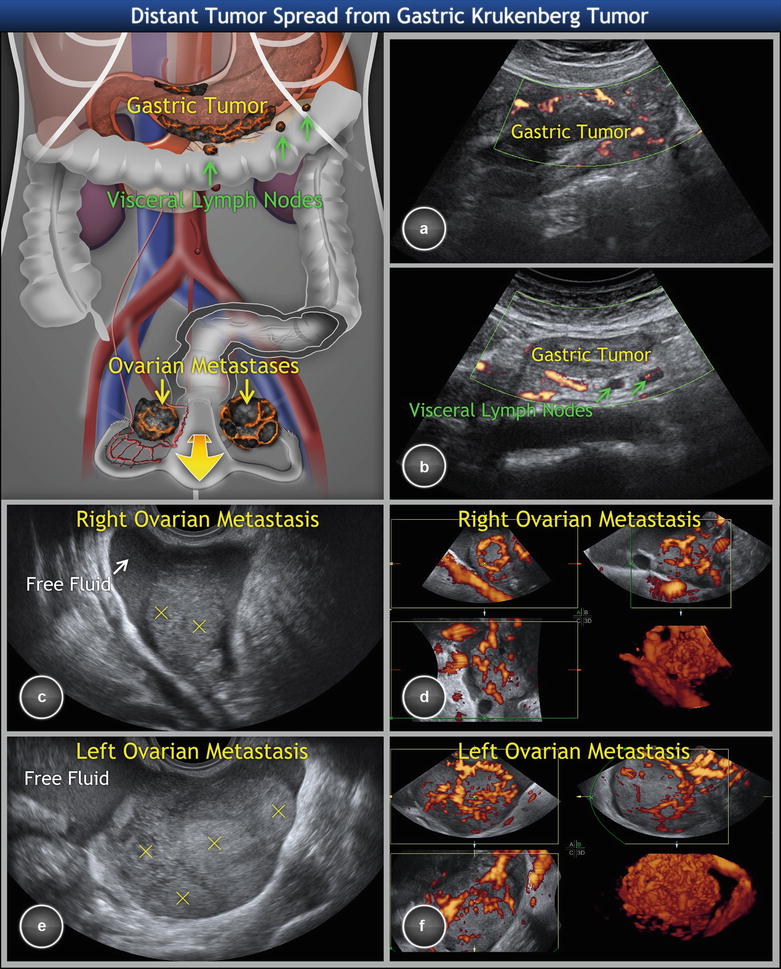
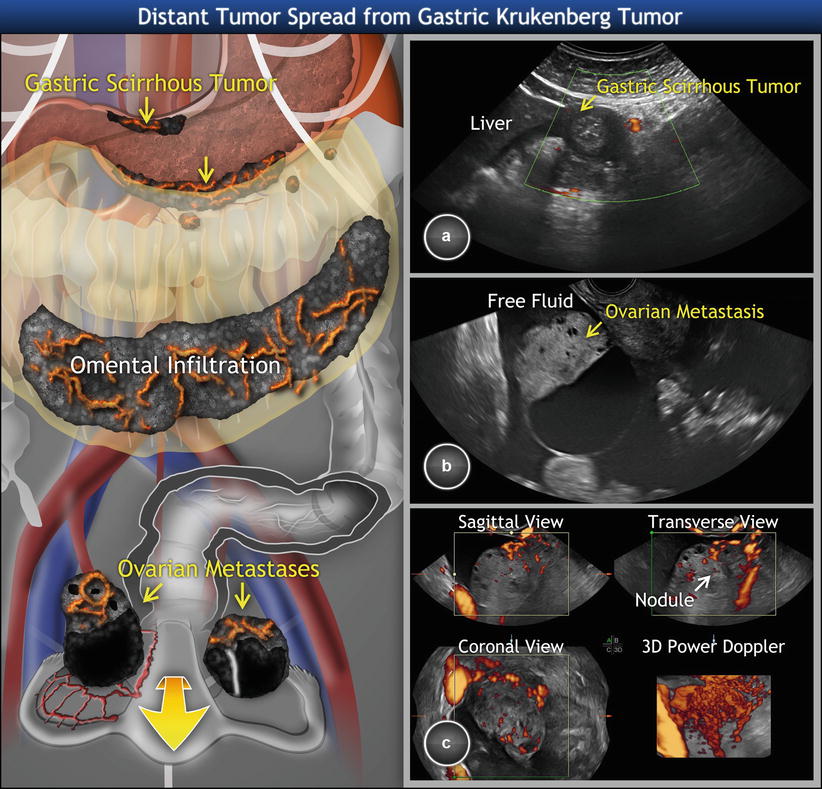

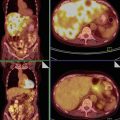
Fig. 5
Metastatic signet ring cell cancer of stomach involving ovaries (Krukenberg tumor). (a) Scirrhous gastric tumor of the greater and lesser curvature of stomach with a rich density of tumor vessels, hypoechogenic wall thickness of more than 5 mm, interrupted layering of the wall, and (b) infiltrated visceral lymph nodes (enlarged, homogeneous, well-circumscribed rounded structures) in the connective tissue surrounding the stomach. (c) Gray-scale and (d) three-dimensional power Doppler of a right ovarian metastasis enclosed in free fluid. (e, f) Similar finding in the left ovarian metastasis. Of note, the transvaginal scan of ovaries shows bilateral solid highly perfused tumors of 6 cm average diameter, with multiple nodules within the tumors (marked with yellow crosses) and demarcated with ring-shaped vessels suggesting metastatic nature. Three-dimensional power Doppler depicts dense, branching, and tortuous vessels with caliber changes and color splashes. Concurrent transabdominal scan revealed primary origin of tumor spread

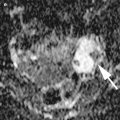
Fig. 6
Metastatic signet ring cell cancer of stomach involving ovaries (Krukenberg tumor). (a) Scirrhous gastric tumor of pylorus with a rich density of tumor vessels (circumscribed widening of the wall with loss of normal wall stratification, luminal narrowing). (b) Gray-scale and (c) three-dimensional power Doppler of a right ovarian unilocular-solid metastasis (size: 5 cm) enclosed in free fluid. The large thin-walled cyst contains fluid of low-level echogenicity. Doppler imaging reveals ring-shaped vessel arrangement around the tumor nodule in solid portion of mass (marked with arrow). The left ovarian tumor visible on schema has two thin-walled cysts separated by relatively small amount of solid tissue. The concurrent transabdominal scan revealed primary origin of tumor spread
On transabdominal scan, the primary source of ovarian Krukenberg tumor is usually found in the pylorus. The sonographic appearance of advanced scirrhous gastric tumor is described in Figs. 5 and 6. Figure 12 (paragraph tumor of the appendix) shows the presence of pelvic and abdominal peritoneal implants of Krukenberg tumor originating in the appendix.
Metastatic Intestinal-Type Adenocarcinoma of the Stomach
These tumors resemble metastatic colon cancer rather than the typical Krukenberg tumor, and their characteristics are described in the paragraph “Intestinal carcinoma.”
In differential diagnosis, an advanced high-grade primary ovarian cancer may sometimes closely simulate metastatic tumors with signet ring cells or intestinal type adenocarcinoma of the stomach involving the ovaries, as documented in Fig. 7. Therefore, the diagnosis of metastatic ovarian tumor and its likely primary source should be always histologically verified (e.g., by tru-cut biopsy of the ovarian tumor and upper endoscopy of stomach).
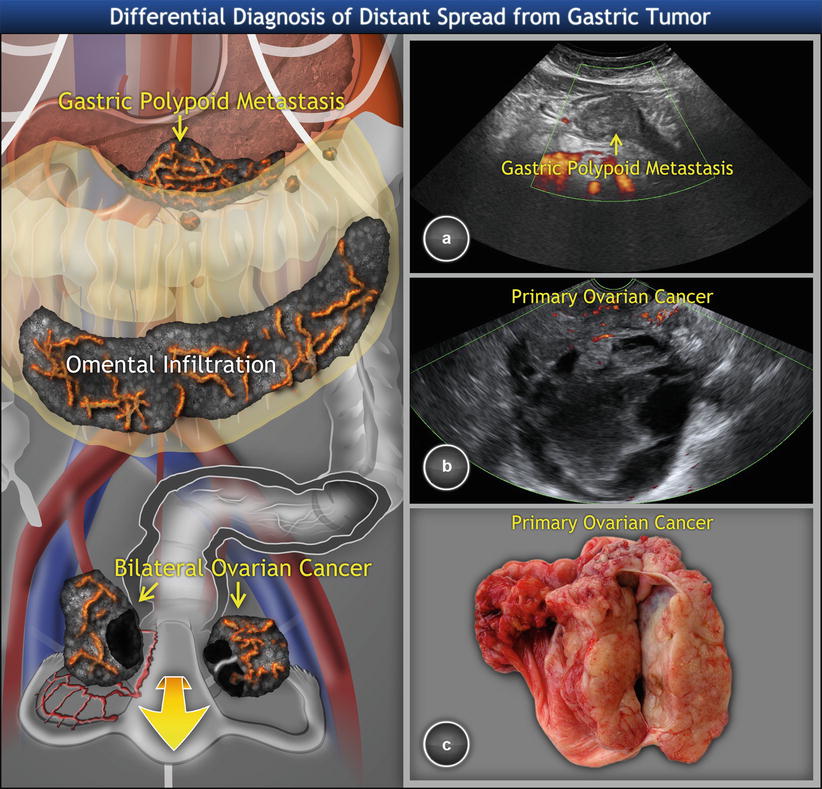

Fig. 7
Metastatic high-grade ovarian cancer involving stomach and disseminating on peritoneal surfaces. (a) Hypoechogenic polypoid tumor in gastric antrum on transabdominal scan. (b) Multilocular-solid ovarian cancer with intracystic fluid of low-level echogenicity and perfused solid component on transvaginal scan. (c) Sectioned tumor showing a mostly smooth-walled cyst surrounded by solid tumor tissue
Intestinal Carcinoma
Most metastatic ovarian tumors of intestinal origin come from the large intestine, with occasional exceptions of small intestine derivation [25]. It was thought that approximately 6 % of women with intestinal cancer have ovarian metastases at some point during the course of their disease [32], but further sectioning of removed ovaries into 2 mm slices increased this frequency to 10 % [33]. Metastases from large intestine to the ovary are seen relatively more frequently in women under 50 years [34]. Predominantly (in 50–75 % cases), the ovarian metastatic disease develops metachronously (following treatment for local-regional colorectal cancer) rather than synchronously and is mainly associated with advanced local tumor extent [25, 35]. In a study by Lash et al., 68 % cases of metastatic colorectal cancers were stage Dukes C [36]. The disease-free interval (time between primary tumor diagnosis and formation of metastatic lesion) is about 3 years [25].
Diagnosis
Clinical symptoms are dependent on the local and/or distant extent of disease. These symptoms include diarrhea/constipation, bloating, cramps, blood in stool, weight loss, iron-deficiency anemia, weakness and fatigue, and symptoms associated with pelvic mass. Relevant tumor markers include carcinoembryonic antigen (CEA) and CA 19–9.
Ovarian metastases from the colon are frequently bilateral (60 % of cases) [28] and may appear on ultrasound as multilocular-solid tumors with intracystic fluid of low-level echogenicity corresponding to mucin. In comparison to Krukenberg tumors or breast carcinoma, the colonic metastases involving ovaries are usually significantly larger [6], with a median of largest dimension being 11 cm (Fig. 8) [36].
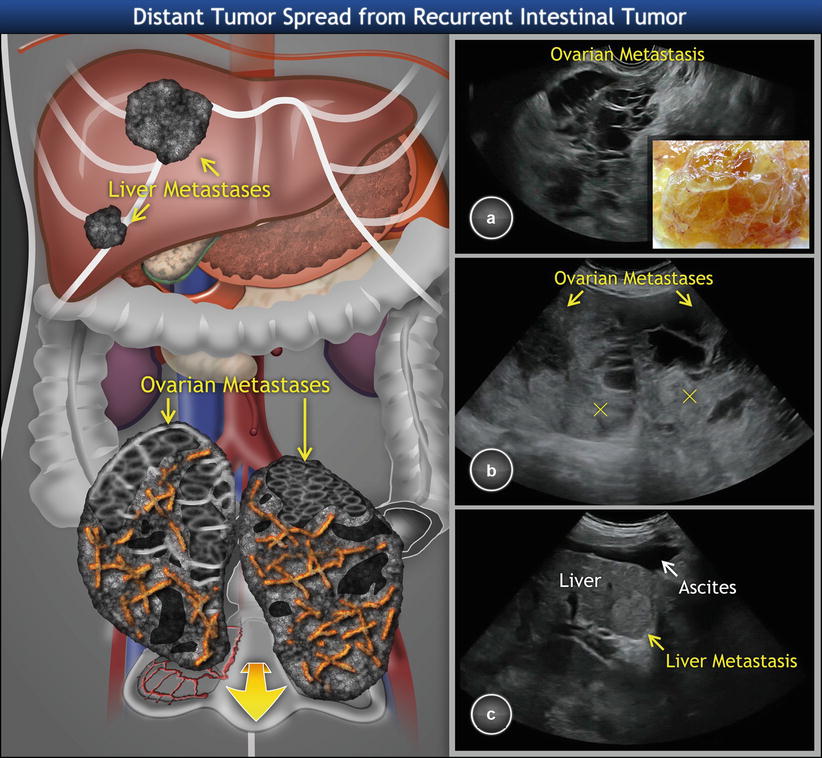

Fig. 8
Recurrent intestinal carcinoma involving the ovaries. (a) Transvaginal scan showing large ovarian multilocular-solid tumor with many small cysts. The tumor fills the whole pelvis. Sectioning reveals yellow tissue composed of multiple thin-walled cysts filled with mucinous fluid (detail). (b) Transabdominal scan depicts large bilateral ovarian tumors of multilocular-solid appearance. The solid component of locular tumors is heterogeneous (marked with crosses). (c) Furthermore, transabdominal scan reveals multiple hyperechogenic liver metastases and ascites resulting from a recurrent colorectal primary tumor
In contrast to primary endometrioid and mucinous ovarian carcinoma, the typical features of metastatic colon cancer involving ovaries are the presence of necrosis in the solid portion, intracystic papillary projections with regular surface, and the presence of parenchymal metastases (Fig. 9) [21].
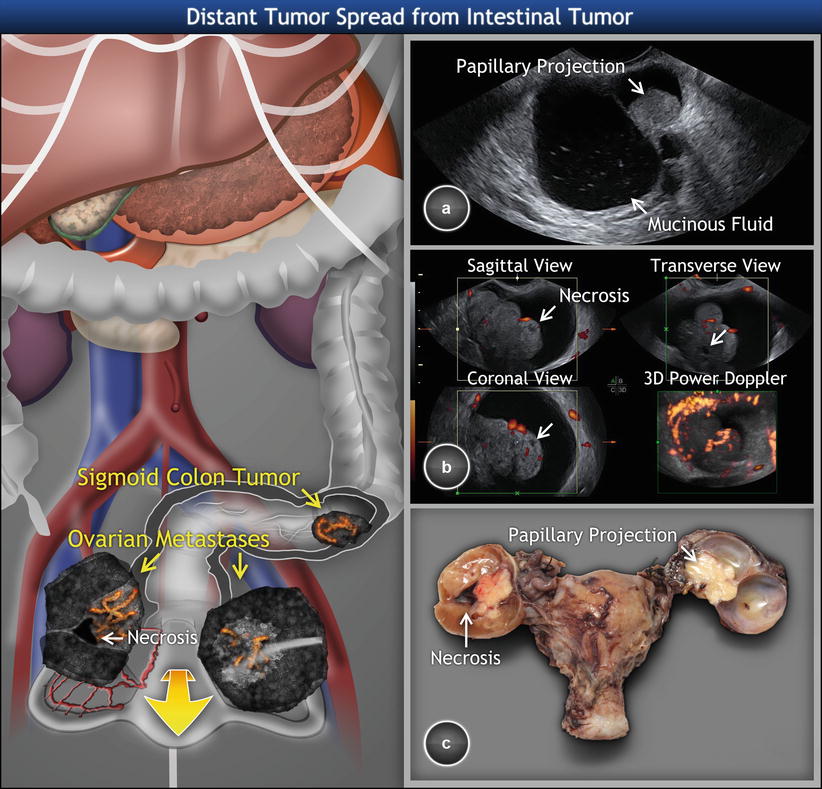

Fig. 9
Sonomorphologic and Doppler pattern of metastatic intestinal tumor involving the ovaries. Colorectal cancer metastasizes to the ovaries in the form of bilateral multilocular-solid large tumors. The intracystic fluid has low-level echogenicity with multiple small echogenic spots (a). The hyperechogenic papillary projection of regular surface is growing from thin hyperechogenic septa as presenting on transvaginal scan of right ovarian metastasis. (b) Three-dimensional power Doppler of the contralateral ovary demonstrating the presence of solid papillary projection with regular surface and tree-shaped vessels entering the papillary projections. The necroses (marked with arrows) present as avascular areas of mixed echogenicity with blurred borders. (c) Sectioned surface of tumors showing mucoid intracystic fluid consistent with primary neoplasm
Because more than two-thirds of large intestine tumors are in the rectum or sigmoid colon, the occult intestinal tumors may be sometimes detected together with ovarian metastases by transvaginal scan (Fig. 10). On ultrasound, colon cancer appears as highly perfused hypoechogenic thickening of one layer or of the whole intestinal wall, with eventual narrowing of the lumen and/or with extraluminal spread (visceral lymph nodes, peritoneal carcinomatosis and ascites, liver metastases). Dilation of intestinal loops with compromised peristalsis proximal to tumor site might be observed.
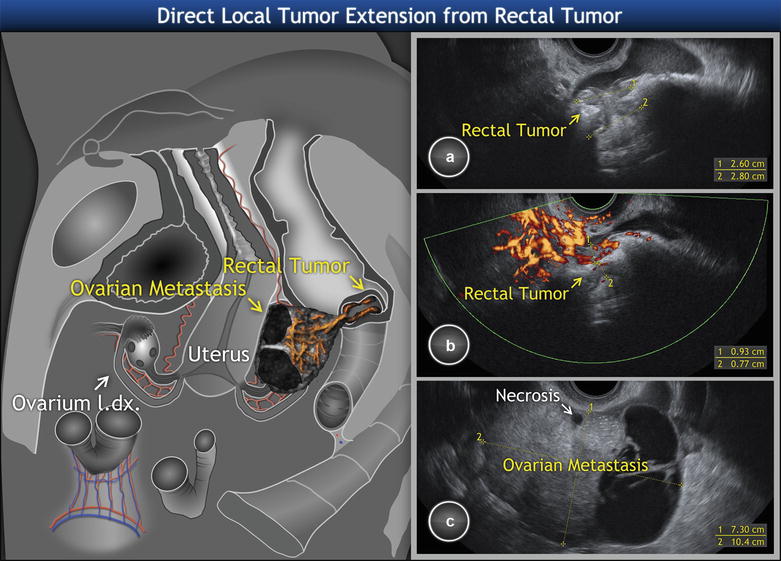

Fig. 10
Unilateral ovarian metastasis from synchronous primary rectal cancer. Transvaginal scan revealing cancerous narrowing of rectal lumen on (a) gray-scale and (b) power Doppler examination. The unilateral ovarian metastatic involvement shows the presence of hypoechogenic necrosis in a solid tissue and intracystic fluid of low-level echogenicity (c). Of note, the tree-shaped architecture of the multiple feeding vessels entering the ovarian tumor
Similarly to gastric tumors, differential diagnosis is difficult here as in some cases neither a gross examination nor any imaging technique could distinguish between a primary colon cancer extending into the ovary and an advanced ovarian cancer infiltrating the colon (Fig. 11). However, this problem may also arise on microscopic examination. The tumors presenting the highest challenge to accurate diagnosis are primary ovarian endometrioid and mucinous adenocarcinomas. Many intestinal metastases to the ovary are misinterpreted as primary ovarian adenocarcinomas on pathologic examination, even when there is known intestinal cancer [37]. In addition to microscopic evaluation, immunohistochemistry can be helpful in distinguishing a typical metastatic carcinoma of large intestine (CK7-/CK20+, CEA+, and CA-125-, estrogen receptor negativity) from a primary endometrioid adenocarcinoma (with opposite immunoprofile), but not from a primary ovarian mucinous tumor.
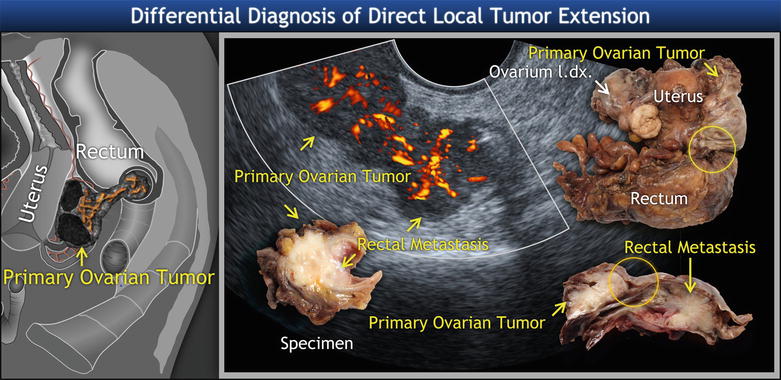

Fig. 11
Metastatic high-grade ovarian cancer protruding into the rectal lumen. Schema and ultrasound image of solid component of ovarian tumors penetrating the rectal wall and growing into the rectal lumen in a polypoid manner, with tree-shaped vessels spreading into the rectal tumor. Biopsy revealed high-grade serous adenocarcinoma originating from ovary. The yellow circle shows the contact of left ovary with rectum on uncut and sectioned specimen
Tumors of the Appendix
Ovarian involvement is most common in cases of low-grade mucinous tumors of the appendix that often have the gross features of a so-called mucocele. Ovarian spread may be also seen in cases of appendiceal invasive adenocarcinomas of the usual intestinal type and typical mucinous type, carcinomas with neuroendocrine differentiation, signet ring cell carcinomas, and others. The ultrasound features of appendiceal intestinal type and mucinous carcinomas and of signet ring cell carcinomas resemble those of similar types arising from the colon and stomach and were already discussed above (Fig. 12).
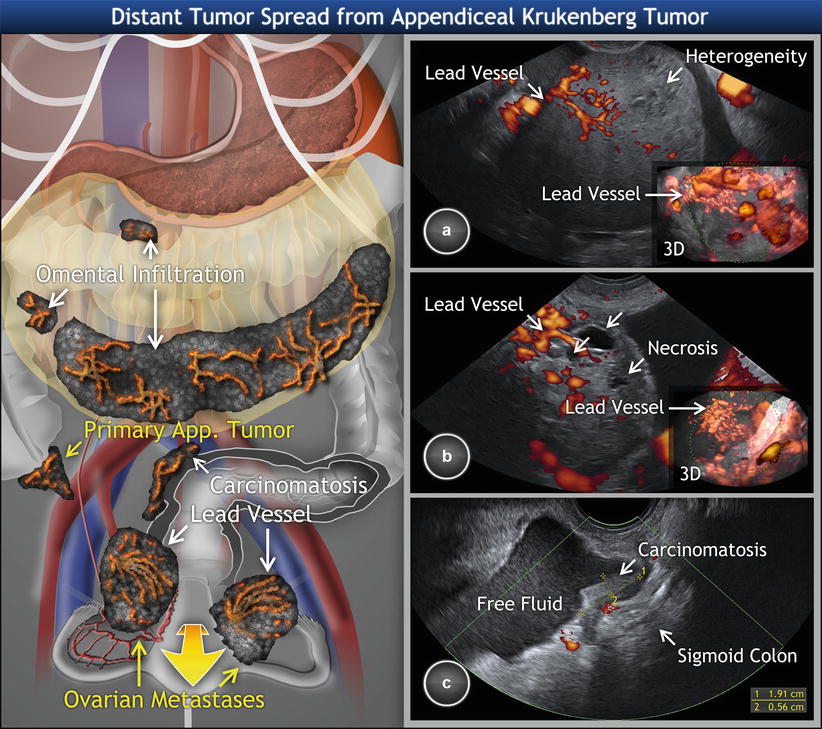

Fig. 12
Metastatic signet ring cell cancer of appendix involving ovaries (Krukenberg tumor). Transvaginal scan showing (a) right and (b) left solid Krukenberg tumor originating in appendix with a typical lead vessel on 2D and 3D ultrasound (detail). Both tumors present with necrosis (arrows) and heterogeneous structure. (c) Krukenberg tumor is accompanied by visceral carcinomatosis (peritoneal implants) on sigmoid colon and free fluid in the pelvis
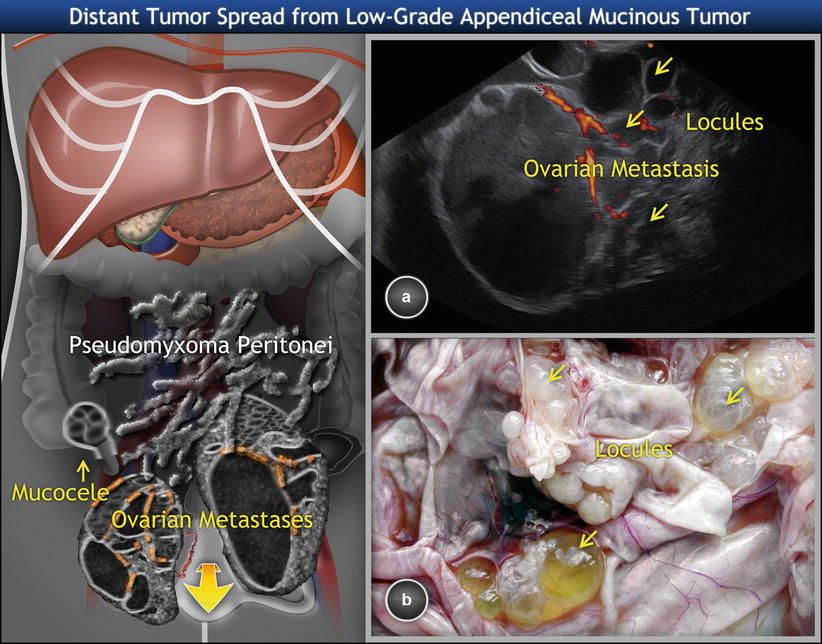
Low-Grade Appendiceal Mucinous Neoplasms
In many cases, ovarian involvement is the first evidence of an appendiceal neoplasm. The association with pseudomyxoma peritonei generally facilitates the recognition of these ovarian tumors as metastatic. For many years, pseudomyxoma peritonei (i.e., the presence of mucinous ascites or mucoid nodules adherent to peritoneal surfaces) was thought to result from ovarian borderline tumors, but it has been recently revealed that virtually all ovarian tumors associated with pseudomyxoma peritonei represent metastases from ruptured primary low-grade mucinous (adenomatous) tumors of the appendix [38]. The rare exception to the gastrointestinal origin of pseudomyxoma peritonei is the occurrence of mucinous tumors arising in ovarian mature cystic teratomas [39, 40].
Diagnosis
The patients are usually middle aged or elderly and typically present with symptoms ascribable to adnexal mass. Other symptoms related to mucocele include pain in the right lower abdominal quadrant, palpable abdominal mass, weight loss, nausea, vomiting, change in bowel habits, anemia, etc. Preoperative CEA and CA19.9 levels are increased in 75 and 58 %, respectively, of patients with pseudomyxoma peritonei [41]. Ultrasound imaging typically shows bilateral ovarian involvement, on average about 16 cm in diameter (Fig. 13) [25].