FIGURE 15-5. Representative IMRT plan for unresectable pancreatic cancer, including axial radiation dose distributions.
(Left panel from Rubin P, Hansen JT. TNM Staging Atlas with Oncoanatomy, 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012.)
1. INTRODUCTION
• Pancreatic adenocarcinoma remains one of the most deadly cancers, with nearly equal incidence and mortality rates. Surgical resection remains the only curative treatment for pancreatic cancer, although even patients who are able to undergo surgery have a poor prognosis. Adjuvant therapy (typically chemotherapy and radiation) modestly improves outcomes in this setting. Unresectable pancreatic cancer is typically treated with definitive chemotherapy and radiation. The utility of intensity-modulated radiation therapy (IMRT) for the treatment of pancreatic cancer is a subject of ongoing research, although given its ability to improve conformality while minimizing radiation dose to critical structures, it may play a role in improving the therapeutic index for treatment of pancreatic cancer in both the adjuvant and definitive settings. This chapter summarizes the anatomy, epidemiology, natural history, diagnosis/staging, prognosis, management, and role of IMRT in pancreatic cancer.
2. ANATOMY
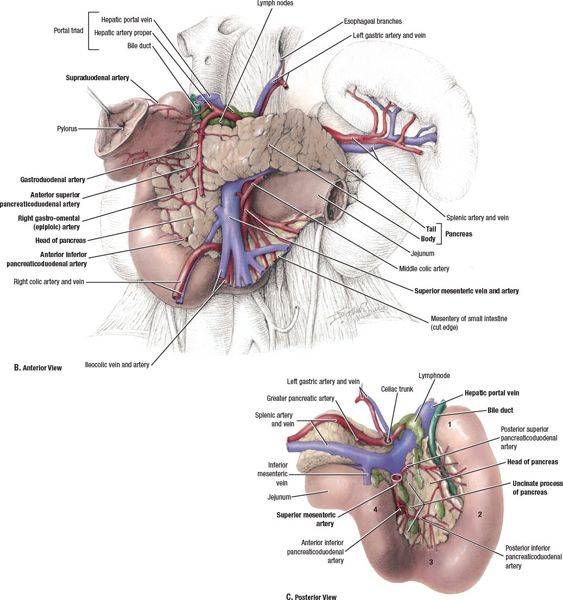
• The pancreas lies in the retroperitoneal space of the upper abdomen, typically at the level of the first two lumbar vertebrae (Fig. 15-1).
• It lies in close vicinity to a number of critical organs, including the stomach, duodenum, jejunum, liver, kidneys, and spleen, as well as major blood vessels, including the celiac artery, superior mesenteric artery/vein (SMA/SMV), splenic artery/vein, portal vein, abdominal aorta, and inferior vena cava.
• The pancreas is divided into four parts—the head (including uncinate process), neck, body, and tail—based on its relation to nearby structures.
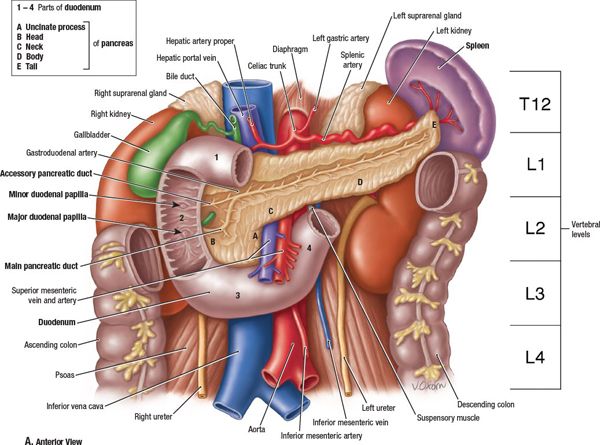
FIGURE 15-1. Anatomy of the pancreas and upper abdomen. The pancreas is conventionally divided into 5 sections based on its relationship to surrounding anatomic structures: the uncinate process, head, neck, body, and tail. (From Agur AMR, Dalley AF, eds. Grant’s Atlas of Anatomy, 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2009, with permission.)
3. EPIDEMIOLOGY
3.1. The U.S. Incidence/Mortality
• In the United States, pancreatic cancer is the ninth most common type of cancer but fourth most common cause of cancer-related deaths.1 Unfortunately, most people diagnosed with pancreatic cancer ultimately succumb to the disease. In 2010, about 43,140 patients were diagnosed with pancreatic cancer, while 36,800 patients died as a result of pancreatic cancer.
3.2. Risk Factors
• Risk factors for pancreatic cancer (with varying degrees of association) include increasing age, male gender, African American race, family history/genetic predisposition (especially among those with Ashkenazi Jewish heritage), tobacco use, obesity/physical inactivity, diet, environmental exposures, chronic pancreatitis, and diabetes mellitus.
4. NATURAL HISTORY
4.1. Histopathologic Differentiation
• The pancreas can give rise to various benign and malignant neoplasms.
• Exocrine pancreatic neoplasms include all tumors that are related to the pancreatic ductal and acinar cells (95% of pancreatic tumors), while endocrine pancreatic neoplasms arise in the endocrine tissues of the pancreas (5% of pancreatic tumors).
• The term “pancreatic cancer” typically refers to exocrine pancreatic adenocarcinomas arising from the ductal epithelium, which represent the vast majority (85%) of pancreatic tumors and is the primary subject of this chapter.
4.2. Patterns of Spread
• Cancer most commonly arises from the pancreatic head, where it typically causes obstruction of biliary drainage. Cancers of the pancreatic head, neck, or body may invade the duodenum, whereas tumors of the pancreatic tail may invade the spleen.
• Primary lymph node drainage includes the superior and inferior pancreaticoduodenal, porta hepatic, celiac, superior mesenteric, and pancreaticosplenic lymph nodes (particularly for tumors of the body/tail). The para-aortic lymph nodes may be involved with more advanced disease.
• The most common sites of distant metastasis include the liver, peritoneum, lungs, and bone.
• In total, approximately 10% have local disease, 30% have regional disease, and 60% have metastatic disease at initial presentation.1
5. DIAGNOSIS AND STAGING SYSTEM
5.1. Symptoms
• The median age of diagnosis in the United States is 72 years. No effective screening methods have as yet been identified.
• The most common symptoms associated with pancreatic cancer include pain (typically along the upper abdomen, the precise location of which relates to tumor location, and potentially radiating to the back), jaundice (including pruritus, acholic stools, and dark urine), and weight loss (with associated anorexia and early satiety). Moreover, patients may have symptoms due to gastric outlet obstruction, pancreatic exocrine insufficiency (resulting in steatorrhea), pancreatic endocrine insufficiency (resulting in new-onset diabetes), pancreatitis, or migratory thrombophlebitis (Trousseau sign). Also, patients may present with a variety of symptoms as a result of metastatic disease to distant organs.
• Clinical symptoms vary according to tumor location. Tumors in the head of the pancreas more often present with jaundice due to early obstruction of biliary flow, whereas tumors in the pancreatic body/tail more commonly present with pain and weight loss. Due to the nonspecific nature of the symptoms associated with tumors of the pancreatic body/tail, cancers in these locations more often present with locally advanced/metastatic disease. Differences between tumors of the pancreatic head and body/tail are summarized in Table 15-1.

5.2. Physical Examination
• The most common abnormal physical findings are secondary to jaundice, which result in abnormal skin color, scleral icterus, and possibly cutaneous excoriations due to pruritis. More rarely, patients may display subcutaneous areas of nodular fat necrosis (pancreatic panniculitis). On abdominal examination, pancreatic cancer may result in an abdominal mass, ascites, or a nontender but palpable gallbladder (Courvoisier sign). Also, one should carefully inspect the liver for signs of involvement, including hepatomegaly or nodularity. In more advanced cases, patients may exhibit signs of cachexia. Other signs of distant spread include left supraclavicular lymphadenopathy (Virchow node), axillary adenopathy (Irish node), periumbilical lymphadenopathy (Sister Mary Joseph node), or a palpable rectal shelf (Blumer shelf), all of which can also be seen as a result of other gastrointestinal malignancies.
5.3. Imaging
• The goal of imaging is to diagnose the tumor, determine the extent of disease spread, and identify patients who may be the candidates for curative surgical resection. Diagnostic evaluation via imaging should precede operative resection/exploration.
• Computed Tomography Scan: Contrast-enhanced, multiphase, multidetector helical computed tomography (CT) scanning with three-dimensional reconstruction of the upper abdomen is the preferred imaging modality for diagnosing/staging pancreatic cancer. Typical CT characteristics of pancreatic cancer include a hypoattenuating mass along with possible secondary signs, such as pancreatic/common bile duct dilation or pancreatic atrophy (Fig. 15-2). Comprehensive CT imaging of the pancreas includes both arterial and venous phase scans to allow assessment of vessel involvement. The lack of major vessel involvement on CT scans is associated with a resectability rate of >90%, whereas partial involvement of major vessels is associated with a 10% to 50% resectability rate.2 CT scans of the abdomen allow for assessment of the size, location, and invasion of the primary tumor as well as, to a lesser degree, regional lymph nodes, and also help to rule out metastasis to common intra-abdominal sites (the liver and peritoneum). CT scans of the chest and pelvis can be obtained to rule out more distant metastasis. Of note, CT of the abdomen is best performed prior to biopsy and biliary stent placement given the difficulty in visualizing pancreatic tumors in the setting of inflammation and metallic artifacts.
• Ultrasound: The initial study in patients who present with jaundice is often transcutaneous abdominal ultrasound. Signs of a pancreatic tumor include dilated bile ducts or the presence of a mass in the head of the pancreas. Endoscopic ultrasound (EUS), on the other hand, may complement CT scanning in terms of assessment of local/regional tumor invasion. It is particularly helpful for assessing local T- and N-staging and for predicting vascular invasion. Moreover, EUS-guided fine-needle aspiration is often the best modality for obtaining a tissue diagnosis, particularly if the tumor is poorly visualized by other imaging modalities. Both transcutaneous ultrasound and EUS are, however, subject to the expertise of the ultrasonographer.
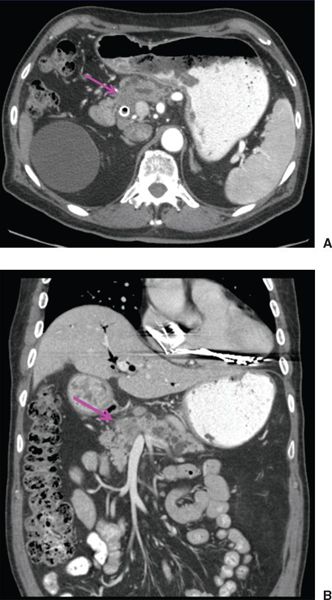
FIGURE 15-2. Axial (A) and coronal (B) computed tomography images of pancreatic cancer. Note hypoattenuating mass in head of pancreas (arrow) with distal pancreatic ductal dilation. Biliary stent is in place.
• Endoscopic Retrograde Cholangiopancreatography: Endoscopic retrograde cholangiopancreatography (ERCP) was once widely used as a diagnostic tool in the past but has since been largely superseded by EUS. Moreover, ERCP is associated with a number of severe complications including pancreatitis, bleeding, and perforation. ERCP may, nonetheless, be indicated should there be equivocal findings on other studies or for therapeutic purposes, as it allows for stent placement in patients who present with cholangitis or who require relief of biliary obstruction.
• Magnetic Resonance Imaging/Magnetic Resonance Cholangiopancreatography: Although magnetic resonance imaging can also detect pancreatic cancer, for the most part, it offers no significant advantage over CT scans.3 Magnetic resonance cholangiopancreatography, on the other hand, may be a useful adjunct to other imaging studies as it allows for better definition of the entire biliary tree and can also identify intrahepatic lesions. Unlike ERCP, it does not require contrast injection and, moreover, it allows for imaging the biliary tree in the setting of disruption/obstruction.
• Positron Emission Tomography: The utility of 18-fluorodeoxyglucose positron emission tomography (PET) in the staging evaluation of suspected pancreatic cancer remains unsettled. In particular, as demonstrated in a previous meta-analysis, it is unclear whether PET offers a benefit beyond that of whole body CT scanning.4 Of note, more recent studies have investigated the utility of integrated PET/CT, which appears more promising.5
• Radiographic Criteria for Resectability/Borderline Resectability: Assessment of resectability is most commonly made based on preoperative CT scans, although findings based on other imaging modalities or at the time of laparoscopy/laparotomy may also be of assistance. Tumors considered to be resectable include those with clear fat planes around the celiac axis, hepatic artery, and SMA, but without evidence of distance metastasis or SMV/portal vein abutment/distortion/thrombus/encasement.6 Although the definition of the “borderline resectable” varies, according to the National Comprehensive Cancer Network7 the criteria for borderline resectable tumors of the head or body include severe unilateral/bilateral SMV/portal vein infringement, less than 180° tumor abutment on the SMA, reconstructible abutment/encasement of the hepatic artery, or short segment SMV occlusion if there is adequate vein above and below the site of tumor involvement to allow for resection and reconstruction, while borderline resectable tumors of the tail include those with less than 180° tumor abutment on the SMA/celiac artery.
5.4. Laboratory Studies
• Routine laboratory tests may reveal a rise in the serum bilirubin (and, with significant liver damage, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) or prothrombin time (PT)/partial thromboplastin time (PTT)), elevation in alkaline phosphatase activity (due to liver dysfunction or bone turnover from metastasis), mild-to-moderate hyperglycemia, anemia, or hypoalbuminemia.
• Several serum markers for pancreatic cancer have been evaluated, however the most clinically useful is carbohydrate antigen 19-9 (CA 19-9). Of note, the ability to produce CA 19-9 requires the expression of Lewis blood antigen and, therefore, this tumor marker is not useful among patients who are Lewis-antigen negative (approximately 10% of the population).8 The reported sensitivity and specificity of CA 19-9 for pancreatic cancer are 80% to 90%.9,10 Given its limited specificity with frequent elevation due to benign pancreaticobiliary disorders, CA 19-9 is not recommended as a screening test.11 Nonetheless, the degree of elevation of CA 19-9 is associated with long-term prognosis and can be used as an early indicator of recurrent disease (in either the nonoperative or operative settings).11–14 Moreover, it can predict the presence of occult metastatic disease among patients undergoing surgery.15
5.5. Biopsy
• Preoperative biopsy of a pancreatic mass can be performed either percutaneously (under CT or ultrasound guidance) or endoscopically (via EUS or ERCP). The former may not be possible if the lesion is not seen on CT and, moreover, is associated with severe, although rare, complications along with at least a theoretical concern of tumor dissemination along the needle path (however, studies suggest the actual risk of this is quite low).16 Whether the addition of molecular genetic analysis to cytologic examination can improve the sensitivity of biopsy is a subject of ongoing research.
• Although biopsy is generally felt to be indicated to confirm diagnosis in the setting of metastatic or unresectable/borderline resectable disease or those who are medically inoperable, the need for preoperative biopsy in a medically fit patient with a potentially resectable, suspicious pancreatic mass is controversial as a negative biopsy does not completely exclude the presence of malignancy. It should be recognized, however, that such an approach is associated with a small risk of radical resection in the setting of a benign lesion, although, of note, the benign process (pancreatitis) most commonly mistaken for pancreatic cancer on the basis of imaging is often suspected based on clinical history.
5.6. Laparoscopy/Cytology
• Although current imaging techniques are accurate in predicting unresectable disease, they are less accurate in predicting resectable disease, due to limited sensitivity to detect small-volume disease (specifically, hepatic or peritoneal lesions measuring less than 1 cm). Staging laparoscopy, with or without laparoscopic ultrasound, may potentially visualize such lesions and therefore reduce the risk of unnecessary laparotomy. Recent studies show that nearly a third of patients thought to be resectable on preoperative imaging will be found to have unresectable disease based on laparoscopic findings.17,18 Nonetheless, the routine use of staging laparoscopy is not universally accepted, with some instead suggesting that it be used in those most likely to harbor occult metastatic disease (patients with tumors measuring >2 cm, tumors in the body/tail, equivocal findings for metastasis on CT, ascites, or subtle clinical or laboratory findings suggestive of metastatic disease).19
• Similarly, the importance of obtaining peritoneal cytology at the time of laparoscopy is a matter of debate. Although most patients with positive peritoneal washings have other findings of metastatic disease, whether positive peritoneal washings as an isolated finding adversely impacts prognosis has not been confirmed.20,21 Of note, the American Joint Committee on Cancer (AJCC) staging system designates positive peritoneal washings as M1 disease.22
5.7. Staging
• Tumors are staged according to the 7th edition of AJCC staging system for carcinoma of the pancreas.22 Tumors are generally classified as resectable (stage I, II), unresectable (stage III), or metastatic (stage IV). Recently, borderline resectable tumors have also been defined radiographically (see above).
• Of note, for patients undergoing surgery, it is prudent to repeat staging studies prior to embarking on potentially toxic adjuvant therapies.
6. PROGNOSTIC FACTORS
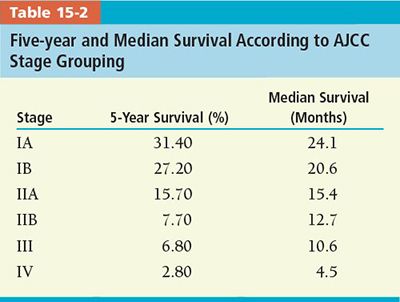
• Stage: T- and N-stages are significant predictors of outcome. Estimated 5-year and median survival according to the AJCC stage groupings is shown in Table 15-2.23
• Surgical Margins: Data suggest that, if taken to surgery, patients with positive resection margins do worse.24 Moreover, in patients with negative surgical margins, the width of the surgical margin may be prognostic, as well.25 It is important to examine not only the pancreatic resection margin but also the retroperitoneal margin as this is often positive, as well.
•
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree