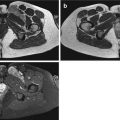
Fig. 6.1
HE, 100×. Schwannomas are well-demarcated lesions surrounded by a fine fibrous capsule (arrow)

Fig. 6.2
Macroscopic image of desmoid tumor (black arrow) infiltrating the surrounding adipose tissue (red arrow). The margins are demarcated with black ink (yellow arrow) (Color figure online)
In most centers fresh frozen material will be stored apart for the Tumor Bank. Subsequently the remaining material will be fixated, embedded in paraffin blocks which will be used for further microscopic, immunohistochemic, and molecular evaluation of the tissue.
6.2.3 Fixation and Its Role to Diagnosis
Adequate fixation of the material plays a crucial role in pathology in order to allow preservation of the cells. Different fixatives have been used such as aldehydes, mercurials, alcohols, oxidizing agents, and picrates. Formaldehyde does not harm the proteins significantly, so that antigenicity is not lost. Therefore, formaldehyde is a good fixative for immunohistochemical techniques. In pathology the most widely used are 10 % and 4 % formaldehyde solutions. The latest has the ability to penetrate the tissue at a rate of 2–4 mm in 24 h [15]. Biopsies and small specimens will be fixated immediately, while bigger specimens have to be cut into thinner slides of maximum 10 mm thickness in order for the fixative to penetrate the tissue. Fixation has to start soon (<30 min) after surgical removal of the tissue, and overfixation (>24–48 h) has to be avoided [16], since this can influence the immunoreactivity of tissue antigens [17]. Also delayed fixation is proven to influence the number of observable mitotic figures in tissues [18, 19].
Once the tissue is fixated, it will be embedded in paraffin and stored in blocks, where the tissue can remain for prolonged periods.
6.3 Role of Frozen Section in Soft Tissue Pathology
Frozen section is one of the most difficult and demanding procedures. It requires well-trained workforce and is costly. The pathologist has to be experienced in the evaluation of soft tissue pathology and to have a close cooperation with the surgeon and the radiologist. Based on a material which has not undergone fixation and without the ability of any ancillary investigation, the pathologist should provide a very specific answer in a limited period of time. The surgeon has therefore first to decide if a frozen section will anyway influence the surgical procedure. If not, the procedure is not indicated [20].
The aim of a frozen section is to evaluate whether the biopsy specimen is representative of the lesion and sufficient enough for further permanent examination. An experienced pathologist can also determine the nature of the lesion, particularly to differentiate between benignity and malignancy. However, specific diagnosis and grading of the lesion depend on permanent sections, and ancillary investigation may also be required for final diagnosis.
Gross specimens submitted for frozen section are examined to evaluate the resection margins in order to intraoperatively determine the level of excision or amputation.
Whenever a metastatic spread of the tumor is suspected radiologically, then a frozen section during surgery could determine whether the lesion is metastatic or not. As expected this requires knowledge of clinical and radiological data, emphasizing once more the need for the collaboration of the different specialties of the multidisciplinary team.
6.4 Tumor Bank
Nowadays, the role of targeted therapy is gaining more and more importance, and individualized therapy is one of the perspectives of cancer research. The aim is to restrict the use of drugs to those patients whose tumor expresses the target, thereby minimizing cost and morbidity. Until today macroscopy, light microscopy, and special techniques in pathology are used to categorize the tumor and determine the stage. Scientists look forward to recognize specific biomarkers for each individual patient that will predict the metastatic risk of his/her disease.
The primary objective of Tumor Bank is to collect high-quality biospecimens and associated clinical data to promote scientific cancer research.
The material that is used for this purpose is fresh tissue that is not needed anymore for pathological examination, as pathology must not be compromised. The tissue is frozen in liquid nitrogen and stored in low temperature. It is very important that the tissue will be proceeded for fixation immediately after removal from the human body, because ischemia can cause degradation of biomarkers. Nevertheless it remains controversial if a prolonged time of cold ischemia after a long warm ischemia is of scientific importance [21]. Studies have indicated that the RNA does not change rapidly after tissues are removed from the body [22–24] and that degradation of RNA happens more extensively at the time of warm ischemia when the vascular supply to the organs is compromised.
When received, the tissue will be frozen in liquid nitrogen and stored in low temperature. Optimal storage temperature is of paramount importance for a high-quality tissue. It is shown that protein activity may persist at low temperatures, even below −80 C [25–31]. Therefore, storage at temperatures at which water particles are still mobile and proteins are still active will result in degradation of the biospecimen [32].
6.5 Role of Light Microscopy in the Diagnosis of Soft Tissue Tumors
6.5.1 Histology
Histology means examination of the tissue on a thin slide stained with hematoxylin and eosin (HE) and remains the gold standard for diagnosis. Histology provides information about the morphology and the architecture of the lesion. Soft tissue tumors in most cases infiltrate diffusely, which is quite consistent for those tumors, in contrast to epithelioid neoplasms, where the cells usually form aggregates. There are of course exceptions to the rules, for instance, a biphasic synovial sarcoma contains also an epithelioid component that is composed of cell groups. On the other hand, an epithelioid neoplasm may also grow diffusely. Some tumors have a very characteristic architecture; examples are solitary fibrous tumor that shows a distinctive “patternless pattern,” schwannoma with also the distinctive Antoni A and Antoni B tissue, representing compact areas alternating with loosely arranged foci of spindle cells (Fig. 6.3) and alveolar rhabdomyosarcoma with discrete nests with discohesive cells. As expected there are atypical forms of these tumors making their diagnosis more difficult and challenging. Of utmost importance is that lymphomas can also infiltrate diffusely; however, lymphomas have other morphological characteristics and a different clinico-radiological presentation.


Fig. 6.3
HE, 200×. Nuclear palisading around fibrillary process (Verocay bodies, arrow) in cellular area of a schwannoma
Cell type can be also recognized on histology. In general soft tissue tumors are composed of cells that are elongated or spindled and have eosinophilic cytoplasm and indistinctive cell borders (Fig. 6.4). The nucleus differs from case to case with the more aggressive and malignant tumors that demonstrate more pleomorphism with enlarged nuclei, abnormal nuclear membrane, and sometimes an evident (macro)nucleolus. Multinucleation may indicate an aggressive cell type, but one has to bear in mind that giant cells are also multinucleated and that some benign tumor cells can also merged into giant forms. Desmoid tumors exhibit at the edge of the lesion multinucleated cells with strong eosinophilic cytoplasm, which is nothing more than degeneration of entrapped muscle cells when the tumor infiltrates between striated muscle (Fig. 6.5).



Fig. 6.4
HE, 100×. DDLPS composed mainly of spindle cells without prominent pleomorphism. Centrally scant lipomatous component

Fig. 6.5
HE, 40×. Desmoid tumor with characteristic infiltrating growth pattern in the surrounding fibro-adipose tissue
Not all soft tissue tumors are composed of spindle cells. Some neoplasms have an epithelioid morphology, such as epithelioid sarcoma, making the diagnosis more complicated.
It is evident that beyond the morphology of the tumor and the cellular composition, there are also other elements that can lead to the diagnosis. Many neoplasms show a vascular pattern that in many cases is diagnostic for this neoplasm. Thus, a “chicken wire” vascular pattern is described in myxoid lipomas and liposarcomas and a hemangiopericytoma-like pattern in solitary fibrous tumor, while schwannomas show hyalinization of the vascular wall.
Histology serves not only in tissue-specific diagnosis but plays also an important role in grading the tumor by recognizing and counting, for instance, the mitotic activity of the cells as well as identifying necrotic areas (Fig. 6.6). On histology one can also estimate the resection margins of a specimen.


Fig. 6.6
HE, 400×. A case of a small blue round cell tumor (in this case alveolar rhabdomyosarcoma) with a central area of necrosis (arrow)
Special histochemical techniques can be used to reveal material in the cell itself as well as in the tumor background (e.g., mucin). In alveolar soft part sarcoma (Fig. 6.7), the cells contain rod-shaped intracytoplasmic crystals that can be easily demonstrated with PAS staining (Fig. 6.8), which is pathognomonic of the lesion.



Fig. 6.7
HE, 200×. Alveolar growth pattern in a case of alveolar soft part sarcoma

Fig. 6.8
PAS, 400×. Alveolar soft part sarcoma with rod-shaped intracytoplasmic crystals (arrow)
6.5.2 Immunohistochemistry
As it is already mentioned, morphology is the gold standard of diagnosis. For many years the diagnosis is made solely on basis of the morphology. It is apparent that many of the subtypes that exist nowadays would not be recognized if there was no possibility of further examination. Immunohistochemistry uses antibodies that are specific to epitopes located on the cells of interest. The antibodies are not specific for malignancy with some exceptions that will be discussed further. That means antibodies indicate the type of cell differentiation in nonmalignant cells but also in tumor cells arising from this type, which will also stain for the same antibody. For instance, endothelial cells stain for CD31; thus, angiosarcomas will stain for the same marker. Combining morphology and immunohistochemistry may allow a more precise diagnosis. However, many tumors present nonspecific positive staining for markers of another differentiation lineage, and unfortunately most antibodies are not specific to one differentiation line. A typical example is S100, an antibody that is widely used in everyday practice. In soft tissue pathology, S100 is known for its strong and diffuse positivity in schwannomas (Fig. 6.9). It stains in neural crest-derived tissue (such as Schwann cells, melanocytes, glial cells), in adipocytes, chondrocytes, dendritic cells, Langerhans cells, macrophages, and myoepithelial and melanocytic cells. This makes things complicated because an undifferentiated tumor with S100 positivity comprises a wide differential diagnosis. Furthermore, tumor cells can lose the antibody expression that reveals the differentiation line, making sometimes the diagnosis impossible or one of exclusion. Less than half of the malignant peripheral nerve sheath tumors (MPNSTs) stain positive for S100, and the staining is usually focal. Diffuse staining for S100 is rarely compatible with conventional MPNSTs and should raise the possibility of other tumors [2].


Fig. 6.9
S100 (DAB), 100×. Strong and diffuse, nuclear, and cytoplasmic staining in all tumoral cells, characteristic of a schwannoma
It is thus of paramount importance to have immunohistochemical markers that are specific and sensitive for the different lines of cell differentiation or for the different tumor types. Nowadays, new antibodies have been added in the armamentarium of a soft tissue pathologist. Some of the most important ones are anti-MDM2 and CDK4. Atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLPS) and dedifferentiated liposarcoma (DDLPS) display amplification of MDM2 and CDK4 genes that are located in chromosome 12q13-15. By immunohistochemistry, overexpression of MDM2 and CDK4 is indicated by nuclear staining for the corresponding antibodies (Fig. 6.10). This positivity is very sensitive for ALT/WDLPS and DDLPS and shows a strong correlation with the gene amplification status [33]. Still, as the majority of antibodies are used in pathology, they are not specific to these entities. It has been shown that intimal sarcomas of pulmonary artery show consistent genetic alteration (gains and amplifications in the 12q13-14 region) and overexpression of the MDM2 gene [34]. Very recently it has been demonstrated that almost half of the MPNSTs exhibit loss of histone H3K27 trimethylation (H3K27me3) which can be highlighted immunohistochemically by loss of nuclear staining for the corresponding antibody. H3K27me3 loss although not very sensitive is a highly specific marker for malignant peripheral nerve sheath tumor, and immunohistochemistry may be useful in differential diagnosis from other high-grade spindle cell sarcomas [35].
There are many novel antibodies with very promising results in defining diagnosis, but one has to be aware of their limitations, as most of them are not entirely specific to one entity or differentiation line.
Jason Hornick et al. separated the novel antibodies into three categories: (1) lineage-restricted transcription factors, (2) protein correlates of molecular alterations, and (3) diagnostic markers identified by gene expression profiling [36], emphasizing on a close correlation of the immunohistochemical profile to the cytogenetic and molecular events in the tumors (Table 6.1) (Figs. 6.9, 6.10, 6.11 and 6.12).



Table 6.1
Immunohistochemical antibodies and most common expression in soft tissue
Immuunohistochemical marker | Soft tissue type |
|---|---|
Myogenin, MyoD1 | Skeletal muscle differentiation |
ERG | Endothelial cells |
Brachyury | Chordoma |
β-catenin | Desmoid tumor |
INI1(loss of expression) | Epithelioid sarcoma |
STAT6 | Solitary fibrous tumor |
DOG1 | GIST |
ALK | Inflammatory myofibroblastic tumor |
MDM2/CDK4 | ALT/WDLPS/DDLPS |
MUC4 | Low-grade fibromyxoid sarcoma |
GLUT1 | Congenital vascular malformation |

Fig. 6.10
MDM2 (DAB), 200×. Core needle biopsy with nuclear immunoreactivity of the cells for MDM2. Case of a WDLPS

Fig. 6.11
Myogenin (DAB), 200×. Nuclear staining in almost every tumor cell in a case of alveolar rhabdomyosarcoma

Fig. 6.12
DOG1 (DAB), 100×. This staining is very specific for GISTs
6.6 Role of Genetics and Molecular Studies in the Diagnoses of Soft Tissue Tumors
6.6.1 Genetic Alterations in Soft Tissue Tumors
Over the last few decades, remarkable advances are made in the understanding of molecular biology of the tumors. This paved the way for improving our diagnostic effectiveness, by defining the underlying genes and the corresponding pathways involved in tumorigenesis. Already in the previous WHO edition of 2002, 11 % of all benign and malignant tumors presented with a karyotypic abnormality, whereas an additional 19 % was also described in the corresponding molecular findings [37].
Many soft tissue tumors harbor a recurrent chromosomal translocation caused by rearrangement of part of genes between nonhomologous chromosomes. This event lead to development of fusion genes which in turn encodes altered proteins that are oncogenic. The most extensively studied translocations relate to Ewing sarcoma. The majority of these sarcomas demonstrate rearrangement between chromosomes 11 and 22, namely, a t(11;22)(q24;q12) [38] or a t(11;22)(q22;q12) [39] rearrangement that results in EWSR1-FLI1 or EWSR1-ERG fusion gene, respectively. The EWSR1 gene located in the q12 domain of chromosome 22 can rarely present translocations with different partner genes located at different chromosomes resulting in a number of other fusion proteins which are also described in Ewing sarcoma [40]. In addition, EWSR1 gene translocations are involved in a variety of non-Ewing sarcomas. For instance, myxoid liposarcoma can show a t(12;22)(q13;q12) reciprocal translocation resulting in a EWSR1-CHOP fusion protein [41].
Another molecular event that is observed in sarcomas is gene amplification. The most illustrative examples are atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLPS) and dedifferentiated liposarcoma (DDLPS). These entities are characterized by amplified sequences in the region q14-15 of chromosome 12 where are located the murine double minute (MDM2) and the cyclin-dependent kinase 4 (CDK4) genes. Co-amplification of those genes represents the hallmark for ALT/WDLPS and DDLPS [42], although recently it has also been described in other entities as well, such as intimal sarcoma to name one [43].
A gene mutation is a permanent alteration in the DNA sequence that makes up a gene. It can affect a single base pair or larger segments of the DNA band. Gastrointestinal stromal tumor (GIST) is a mesenchymal neoplasm that is believed to arise from or is differentiated toward interstitial cell of Cajal. It is proven that these neoplasms can harbor a somatic oncogenetically activating mutation. The vast majority demonstrate KIT (which is a proto-oncogene receptor tyrosine kinase) mutations on exon 11. This can be an insertion, a deletion, or a missense mutation. After this initial discovery, three other less frequent hot spots came into light, regarding exons 9, 13, and 17 [44]. PDGFRA gene encodes platelet-derived growth factor receptor A. A small percentage of GIST that does not show KIT mutations can demonstrate a PDGFRA mutation. The exons involved in this case are 12, 14, and 18 with the last being the most common one [44]. Both KIT and PDGFRA are driver mutations and are presumed to be the initiating oncogenic event. These mutations are mutually exclusive, namely, when one happens, the other is not present. The overall mutation frequency for KIT and PDGFA is 86 % with 14 % being wild type. Nowadays it is known that many of those wild-type GISTs contain another mutation, with most extensively described the BRAF V600E mutation [44].
From a genetical point of view, sarcomas are divided in two groups. First is the group with a simple karyotype that presents one main genetic alteration. As previously described, this alteration can be either a somatic mutation, or a gene amplification, or a recurrent translocation, or even an intergene deletion. Second are those presenting with more complex karyotypes [37, 45–48]. This last group represents almost two-thirds of soft tissue sarcomas and shows aberrant chromosomal events but no recurrent reciprocal translocations. Most demonstrate mutations involving p53 gene and retinoblastoma gene [45, 46, 48].
6.6.2 Molecular Techniques in Clinical Practice
Three main technical approaches are nowadays used in clinical practice regarding soft tissue tumors. Those are conventional cytogenetic analysis, fluorescence in situ hybridization (FISH) and Reverse Transcription Polymerase Chain Reaction (RT-PCR) [37, 45].
Conventional cytogenetic analysis or simply karyotyping aims to detect numerical and/or large structural chromosome abnormalities in metaphase cells. Both primary and secondary changes can be identified. This study is limited to fresh, not fixated, sterile tumor tissue and demands special culture for the tumor cells to grow.
FISH detects the presence, absence, relative positioning, and/or the copy number of specific DNA segments by fluorescence microscopy. It can be performed on either fresh or formalin-fixed and paraffin-embedded (FFPE) tissue. In contrast to the karyotyping, this method requires the knowledge of the specific target examined. FISH testing uses dual-color/fusion or dual-color/break-apart probes to detect specific rearrangements, involving a variety of translocation events (Fig. 6.13). There are also the locus-specific probes coupled with a control probe usually pointing the centromere of the chromosome and aim to identify gene amplifications or losses.


Fig. 6.13
FISH by synovial sarcoma. SYT break-apart probe, showing splitting of red and green signals (By Dr. Karen Zwaenepoel, biomedical scientist, department of pathology, University Hospital of Antwerp) (Color figure online)
RT-PCR uses specific primers to copy or amplify a small section of a DNA or RNA sequence. It is quick and simple and can be performed on either snap-frozen or FFPE material. Its role in soft tissue pathology is to identify chimeric or fusion genes as well as oncogenic mutations.
Next-generation sequencing (NGS) takes nowadays more and more part in molecular biology of human tissue. It is a high-throughput DNA sequencing technique that aims to investigate simultaneously in the same specimen a large number of mutation genes that are proven to cause tumorigenesis. It can be performed on FFPE tissue which makes this technique even more applicable. The advantage of this technique is that different genetic aberrations can be tested simultaneously. This technique requires high-quality DNA/RNA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree