The ocular motor nerves supply motor fibers to the extraocular muscles of the globe and levator muscle of the eyelid and parasympathetic pupillomotor fibers to the ciliary ganglion. Lesions are classified according to the anatomic location at which they occur: nuclear, fascicular, cisternal, cavernous, or orbital apex. The course each nerve follows influences the sites at which it is most vulnerable to damage and the pathologies to which it is exposed. Cranial nerve palsies frequently are associated with neurologic deficits that may assist in localizing the lesion anatomically. This article provides an overview of the pathology of the ocular motor nerves.
The ocular motor nerves are cranial nerves three (CNIII), four (CNIV), and six (CNVI), also known as the oculomotor, trochlear, and abducent nerves, respectively. Collectively they supply motor fibers to the extraocular muscles of the globe and levator muscle of the eyelid and parasympathetic pupillomotor fibers to the ciliary ganglion.
Lesions of the ocular motor nerves are classified according to the anatomic location at which they occur: nuclear, fascicular, cisternal, cavernous, or orbital apex. The course each nerve follows influences the sites at which it is most vulnerable to damage and the range of pathologies to which it is exposed. Although cranial nerve palsies may occur in isolation, they frequently are associated with additional neurologic deficits that may assist in localizing the lesion anatomically.
This article provides an overview of the pathology of the ocular motor nerves with an emphasis on lesion localization and the associated imaging findings.
Oculomotor nerve
The oculomotor nerve (CNIII) innervates the superior, medial, and inferior recti, inferior oblique, and levator palpebrae superioris muscles. In addition, the nerve carries preganglionic parasympathetic pupillomotor fibers to the ciliary ganglion. In two large series, the most common identifiable causes of CNIII palsy in order of frequency were ischemia (predominantly microvascular), trauma, aneurysm, and neoplasia.
Nuclear Lesions
The nuclear complex of CNIII lies within the periaqueductal midbrain at the level of the superior colliculus. Complete nuclear injuries result in contralateral superior rectus paresis, bilateral ptosis, ipsilateral dilatation of the pupil (mydriasis), and ipsilateral paresis of inferior oblique and the medial and inferior recti. Lesions also may be incomplete with sparing of pupil function in caudal lesions and absence of ptosis in rostral lesions.
Fascicular Lesions
Axons projecting from the nuclear complex coalesce to form fascicles that pass anteriorly through the midbrain. Fascicular lesions usually involve adjacent structures, and the association of third nerve palsies with additional neurologic signs gives rise to several eponymous syndromes. Injury to the cerebral peduncle produces a contralateral hemiparesis (Weber’s syndrome); injury to the red nucleus produces a contralateral tremor (Benedikt’s syndrome); and damage to the superior cerebellar peduncle gives rise to ipsilateral ataxia (Nothnagel’s syndrome).
Cisternal Lesions
After emerging from the ventral surface of the midbrain, CNIII passes between the posterior cerebral artery (PCA) and the superior cerebellar artery (SCA). It then courses inferior to the posterior communicating artery (PcomA) and inferomedial to the uncus of the temporal lobe to enter the cavernous sinus. Complete peripheral CNIII palsy manifests clinically as ptosis, mydriasis, and deviation of the eye downwards and outwards. Injury to the nerve also may result in partial dysfunction, sparing pupil function or affecting only a single extraocular muscle.
Cavernous Sinus and Orbital Apex Lesions
Within the cavernous sinus, CNIII lies superiorly and laterally, just deep to the dura propria, adjacent to CNIV. It passes anteriorly and exits the cavernous sinus at the orbital apex. Pathologies affecting the cavernous sinus and orbital apex commonly give rise to multiple cranial nerve palsies, often in combination with dysfunction of the traversing vascular structures. At the extreme end of this spectrum are cavernous sinus syndrome and orbital apex syndrome. Cavernous sinus syndrome is characterized by ophthalmoplegia, chemosis, proptosis, Horner’s syndrome (due to involvement of occulosympathetic fibers around the internal carotid artery [ICA]), and trigeminal pain or sensory loss. Orbital apex syndrome is characterized by ophthalmoplegia, optic neuropathy, and involvement of the ophthalmic division of the trigeminal nerve (CNV). As the cavernous sinus and orbital apex are continuous, the etiologies of the syndromes are similar and clinical features may overlap.
Trochlear nerve
The trochlear nerve (CNIV) innervates the superior oblique muscle. Lesions of the nerve present clinically with vertical diplopia that worsens in downgaze. The most common identifiable cause of CNIV palsy is trauma, followed by ischemia and neoplasia.
Nuclear Lesions
The nucleus of CNIV lies within the midbrain at the level of the inferior colliculus, caudal to the nuclear complex of CNIII. Nuclear lesions give rise to contralateral superior oblique weakness. Isolated nuclear lesions are rare, however, and associated findings include ipsilateral internuclear ophthalmoplegia (injury to the medial longitudinal fasciculus [MLF]), ipsilateral Horner’s syndrome (injury to sympathetic fibers within the periaqueductal gray matter), and contralateral afferent papillary defect (injury to the superior colliculus). More extensive lesions may result in signs of dorsal midbrain (Parinaud’s) syndrome.
Fascicular Lesions
The fascicles run posteriorly, decussate within the superior medullary velum, and exit the dorsal midbrain immediately below the inferior colliculus. Fascicular lesions arising after the decussation result in an ipsilateral superior oblique weakness.
Cisternal Lesions
The cisternal segment courses around the lateral midbrain within the quadrigeminal and ambient cisterns. Because of its long course and relation to the edge of the tentorium, the cisternal segment of CNIV is particularly vulnerable to injury. Lesions may be associated with contralateral hemiparesis resulting from compression of the cerebral peduncle or contralateral ataxia resulting from compression of the superior cerebellar peduncle.
Cavernous Sinus and Orbital Apex Lesions
CNIV lies within the lateral wall of the cavernous sinus inferior to CNIII and superior to the ophthalmic division of CNV. In common with the other ocular motor nerves, lesions of CNIV frequently are associated with multiple cranial nerve palsies as part of the spectrum of cavernous sinus and orbital apex syndromes.
Trochlear nerve
The trochlear nerve (CNIV) innervates the superior oblique muscle. Lesions of the nerve present clinically with vertical diplopia that worsens in downgaze. The most common identifiable cause of CNIV palsy is trauma, followed by ischemia and neoplasia.
Nuclear Lesions
The nucleus of CNIV lies within the midbrain at the level of the inferior colliculus, caudal to the nuclear complex of CNIII. Nuclear lesions give rise to contralateral superior oblique weakness. Isolated nuclear lesions are rare, however, and associated findings include ipsilateral internuclear ophthalmoplegia (injury to the medial longitudinal fasciculus [MLF]), ipsilateral Horner’s syndrome (injury to sympathetic fibers within the periaqueductal gray matter), and contralateral afferent papillary defect (injury to the superior colliculus). More extensive lesions may result in signs of dorsal midbrain (Parinaud’s) syndrome.
Fascicular Lesions
The fascicles run posteriorly, decussate within the superior medullary velum, and exit the dorsal midbrain immediately below the inferior colliculus. Fascicular lesions arising after the decussation result in an ipsilateral superior oblique weakness.
Cisternal Lesions
The cisternal segment courses around the lateral midbrain within the quadrigeminal and ambient cisterns. Because of its long course and relation to the edge of the tentorium, the cisternal segment of CNIV is particularly vulnerable to injury. Lesions may be associated with contralateral hemiparesis resulting from compression of the cerebral peduncle or contralateral ataxia resulting from compression of the superior cerebellar peduncle.
Cavernous Sinus and Orbital Apex Lesions
CNIV lies within the lateral wall of the cavernous sinus inferior to CNIII and superior to the ophthalmic division of CNV. In common with the other ocular motor nerves, lesions of CNIV frequently are associated with multiple cranial nerve palsies as part of the spectrum of cavernous sinus and orbital apex syndromes.
Abducent nerve
The abducent nerve (CNVI) innervates the ipsilateral lateral rectus muscle, which serves to abduct the globe, and palsies present clinically with horizontal diplopia. It is the ocular motor nerve that is injured most frequently. The most common identifiable cause of CNVI palsy is ischemia, followed by trauma, neoplasia, and aneurysm.
Nuclear Lesions
The nucleus of CNVI lies just beneath the floor of the fourth ventricle in the dorsal pons. It lies close to the MLF and the fascicles of the facial nerve (CNVII) course around its dorsal aspect producing a protruberance, known as the facial colliculus. Lesions of CNVI nucleus produce a conjugate horizontal gaze palsy toward the side of the lesion. Lesions usually are associated with additional neurologic signs, the most common of which is an ipsilateral CNVII palsy resulting from facial colliculus injury. Injury to the MLF results in a one-and-a-half syndrome characterized by loss of conjugate gaze toward the lesion and reduced adduction away from the lesion.
Fascicular Lesions
The fascicles pass anteriorly through the pons to emerge from its caudal aspect. Lesions affecting CNVI fascicles usually are associated with features reflecting more widespread pontine injury. Lesions of the anterior paramedian pons result in Millard-Gubler syndrome characterized by ipsilateral CNVI and CNVII palsies and contralateral hemiplegia. Ischemic lesions in the territory of the anterior inferior cerebellar artery (AICA) may result in a combination of ipsilateral CNV, CNVI, and CNVII palsies with deafness and Horner’s syndrome (Foville’s syndrome).
Cisternal Lesions
The cisternal portion of the nerve passes rostrally, paralleling the clivus ventrally. It then passes into the fibro-osseous tunnel, known as Dorello’s canal, passing over the petrous apex to enter the cavernous sinus. This segment of CNVI is particularly vulnerable to pathologies arising from the skull base or from compression against the clivus or basilar artery (BA) and to pathologies affecting the petrous apex.
Cavernous Sinus Lesions
Because of its close contact with the internal carotid artery and surrounding sympathetic plexus, the combination of a CNVI palsy with occulosympathetic dysfunction should localize the lesion to the cavernous segment of the nerve.
Ischemia
Ischemia, including diabetic ophthalmoparesis, is the most common identifiable cause of CNIII and CNVI palsies and is second only to trauma in CNIV palsies. Overall, ischemia accounts for approximately a quarter of cases of ocular motor nerve palsy in which underlying cause can be identified.
Nuclear/Fascicular
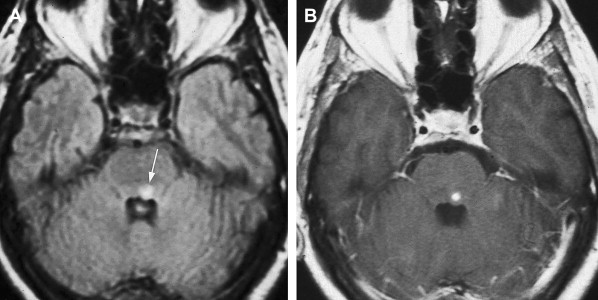
The mesencephalon is supplied by branches of the upper BA, SCA, and PCA. It frequently is affected by posterior circulation embolic stroke but usually with simultaneous involvement of the pons, thalamus, or cerebellum. Isolated infarctions of the midbrain are unusual, but when they occur, ocular motor disturbance, usually in the form of CNIII palsy, is the second most common feature after ataxia. As expected, such infarctions usually are localized to the anteromedial part of the brainstem where the nucleus and fascicles of CNIII are situated. Occasionally, infarctions may be localized enough to result in isolated ocular motor nerve palsies ( Fig. 1 ). As discussed previously, AICA territory infarctions may result in Foville’s syndrome.
Extra-Axial
Microvascular infarction of the cisternal segments of CNIII, CNIV, and CNVI, secondary to systemic vascular diseases, such as diabetes and hypertension, is the most common overall cause of ocular motor nerve dysfunction.
Involvement of CNIII usually results in a pupil sparing palsy. This can be explained by the parasympathetic pupilloconstrictor fibers distributed in an arc sited dorsomedially within the periphery of the nerve; it is the more central neurons supplied by end arteries from the vasa vasorum that are most vulnerable to ischemia.
MRI appearances of the nerve after microvascular infarction almost always are normal, although moderate enhancement of the cisternal portion has been described.
Aneurysms, neurovascular conflicts, and fistulas
In addition to lesions resulting from ischemic vascular disease, ocular motor nerve dysfunction may arise because of compression by vascular structures, in particular intracranial aneurysms, or secondary to carotid-cavernous fistulas.
Cisternal
Intracranial aneurysms and dolichoectatic vessels
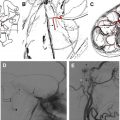
Intracranial aneurysms and dolichoectatic vessels account for up to 14% of CNIII palsies overall and a much higher proportion of isolated peripheral palsies. CNIV and CNVI are affected less frequently accounting for approximately 2% and 4% of cases, respectively. The vascular structures that potentially may give rise to ocular motor dysfunction are determined by the anatomic course of the cisternal segments of each nerve.
On route to the cavernous sinus, CNIII first passes between the PCA and SCA and then inferior to the PcomA. Although palsies related to aneurysms of the SCA are well recognized ( Fig. 2 ), aneurysms of the PcomA ( Fig. 3 ) are the most common culprit, with the incidence of a CNIII deficit at presentation ranging from 34% to 61%. In cases of ruptured aneurysm, neural injury occurs secondary to direct hemorrhagic dissection of the nerve or uncal herniation. In cases of unruptured aneuryms, localized extravasation leading to intraneural fibrosis or acute dilatation of the aneurysm sac leading to mechanical distortion and edema of the nerve are the proposed mechanisms.
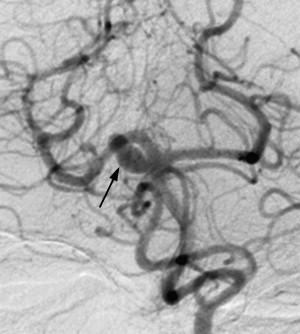
Most series demonstrate that a high proportion of isolated CNIII palsies secondary to PcomA aneurysms are associated with disturbance of pupillomotor function. Anatomically, this is explained by the aneurysms tending to point posteriorly, inferiorly, and laterally, making the peripheral, dorsomedially sited parasympathetic pupillomotor fibers most vulnerable to impingement.
The discrimination between an acute onset of isolated CNIII palsy resulting from aneurysm and resulting from other causes, principally microvascular infarction, is critical. Microvascular infarction follows a benign self-limiting course with almost 100% recovery. In contrast, nerve dysfunction secondary to aneurysm may herald a more sinister outcome and it is shown that the rupture rate of aneurysms associated with CNIII palsy is 2.4 times that of aneurysms without. Most studies concur that the most important discriminatory feature clinically is the presence or absence of pupillary involvement. Between 68% and 80% of CNIII palsies resulting from microvascular infarction spare the pupil compared with 4% to 14% of those resulting from aneurysm. Another potentially useful discriminatory feature is the presence of aberrant CNIII innervation resulting from misdirected regrowth of fibers to an inappropriate target structure. This process takes at least 8 weeks and suggests a compressive lesion, such as an unruptured aneurysm or tumor, almost never occurring after microvascular infarction. Retro- or periorbital pain is associated with both causes and is not a useful discriminatory feature.
As CNIV passes anteriorly between the lateral midbrain and tentorial edge, it also passes between the PCA and SCA. In contrast to CNIII, CNIV nerve palsy secondary to aneurysms of these vessels is rare.
CNVI courses through the prepontine cistern in close proximity to the vertebral artery (VA) and BA. As a result, the most common vascular causes of cisternal segment dysfunction are dissecting aneurysms of the VAs or dolichoectatic vessels.
Although digital subtraction angiography (DSA) remains the gold standard investigation, CT angiography and magnetic resonance angiography (MRA) are demonstrated as having sensitivities approaching that of DSA for detecting aneurysms 5 mm or more in diameter. In the context of aneurysms presenting with CNIII palsy, it is estimated that MRA detects 98% of aneurysms and overlooks only 1.5% of those that subsequently rupture.
Neurovascular conflicts
Neurovascular conflicts are disorders believed to arise from vascular impingement and are characterized by hyperactive nerve dysfunction rather than nerve palsy. This group includes disorders, such as trigeminal neuralgia (CNV), hemifacial spasm (CNVII), and superior oblique myokymia (SOM) (CNIV). A proposed common mechanism for the neurovascular conflicts group is vascular compression at a specific portion of the cisternal segment of the nerve known as the root exit zone (RExZ). The cisternal portion of the ocular motor nerves can be divided into a central (glial) segment sheathed in myelin derived from oligodendrocytes and a peripheral segment sheathed in myelin derived from Schwann cells. The RExZ refers to this central portion and is less stable anatomically and electrophysiologically because it lacks perineurium and epineurium. It is proposed that the region of transition between oligodendrocyte and Schwann cell–derived myelin is particularly vulnerable to continued pulsatile pressure, which may result in focal demyelination and short-circuiting of impulses. The length of the RExZ differs for each nerve, measuring up to 4 mm for CNIII, 1 mm for CNIV, and 1 mm for the CNVI.
Neurovascular conflicts involving CNIII usually are the result of atherosclerotic, dolichoectatic, or abnormally positioned PCA or SCA. Neurovascular conflicts of CNIV occur because of the SCA or its branches lying in close contact with the nerve within the ambient cistern. Compression of the CNIV RExZ may lead to a rare recurring disorder of ocular motility, called SOM ( Fig. 4 ). SOM is characterized by unilateral paroxysmal oscillopsia and microtremor resulting from intermittent contractions of the superior oblique muscle.
In microsurgical and radiologic studies the vessels that are identified as having a close relationship with CNVI are the AICA, posterior inferior cerebellar artery (PICA), BA, VA, SCA, and the petrosal vein. The VA, in particular, is reported as causing CNVI compression.
Neurovascular contacts within the central segment of the ocular motor nerves, however, commonly occur in asymptomatic individuals, and arteries may even penetrate the nerve without clinical consequences.
MRI is the most useful imaging tool for imaging neurovascular conflict syndromes. Although imaging of CNIII is relatively easy because of its larger diameter (2.5 to 3 mm), CNIV and CNVI are smaller (diameter 0.75 to 1 mm for CNIV and 0.43 to 1.85 for CNVI), and imaging the cisternal segments is more challenging. Furthermore, CNIV lies in close proximity to vessels of similar course and caliber in the ambient cistern, making depiction particularly difficult. The ocular motor nerves are identified best using high-resolution imaging techniques, such as the 3-D constructive interference in steady state (CISS) sequence, 3-D T2-weighted (T2-w) fast spin-echo, or 3-D fast imaging employing steady-state acquisition sequences. The 3-D datasets of these sequences allow reconstructions along each individual nerve course and very thin slices (down to 0.6 mm). The problem of misinterpretation of a vascular structure as a nerve can be overcome by adding a 3-D time-of-flight sequence, with and without administration of gadolinium-DTPA. Furthermore, this technique allows differentiation of arteries and veins.
Cavernous
Aneurysms
Aneurysms of the distal ICA may occur extradurally within the cavernous sinus ( Fig. 5 ). The most common symptom associated with such aneurysms is ocular motor dysfunction. Unlike intradural aneurysms, the nerve affected most commonly is CNVI, possibly related to its anatomic location within the central venous portion of the sinus tethered to the ICA. Frequently, there is evidence of a more extensive cavernous sinus syndrome with multiple ocular motor palsies, trigeminal pain, and Horner’s syndrome.
Carotid-cavernous fistulas
Carotid-cavernous fistulas represent abnormal shunting of arterial blood through the cavernous sinus and most commonly are secondary to rupture of extradural cavernous ICA aneurysms or trauma. They are categorized as direct or indirect depending on whether or not the arterial shunt is derived from the ICA itself or arteries supplying the dura of the cavernous sinus. Typically, patients who have the direct type present with chemosis, pulsatile exophthalmos, and ocular bruit, whereas those who have indirect type present with a more insidious onset of exophthalmos, glaucoma, and pain. Ophthalmoplegia is a common finding in both types and in one series examining indirect fistulae, isolated ocular motor dysfunction was found the presenting feature in one third of patients. Moreover, recovery rate of ocular motor function after treatment of the fistula was more than 50% at 1 year.
The sensitivity of CT in detecting carotid-cavernous fistulas is low, but dilatation of the cavernous sinus and superior ophthalmic vein, proptosis, and expansion of the extraocular muscles may be demonstrated. MRI is more sensitive and, in addition to the CT features, flow-related enhancement of the cavernous sinus with abnormal flow voids within the superior ophthalmic vein or petrosal veins may be visible. DSA is the most sensitive imaging modality and the diagnosis is made with visualization of arterial shunting to the cavernous sinus from the ICA or dural arteries ( Figs. 6 and 7 ).
Trauma
Ocular motor nerve dysfunction is a common finding after severe head trauma and may be seen after minor head injuries. CNVI is the ocular motor nerve damaged most frequently, trauma being the second most common cause of injury to this nerve overall. In addition, trauma is the most common identifiable cause of CNIV palsy and the second most common cause of damage to CNIII.
The ocular motor nerves may be damaged as part of the primary injury at the moment of the traumatic event or may be damaged secondarily by swelling and transcompartmental herniation of brain structures.
Nuclear/Fascicular
The pons and midbrain are susceptible to primary injury directly from impact against the tentorial incisura and indirectly from diffuse axonal injury. The nucleus of CNIV is supplied by paramedian branches of the basilar bifurcation. These very fine vessels are susceptible to shearing forces leading to secondary ischemic infarction.
Cisternal
Primary injuries
The cisternal portions of the ocular motor nerves are particularly vulnerable at points of relative fixation.
CNIII has the shortest cisternal course of the ocular motor nerves. Traumatic injury to this segment occurs as a result of differential movement between the brainstem and supratentorial structures or skull base leading to rootlet avulsion or stretching over the tough posterior petroclinoid ligament.
CNIV has the longest cisternal course. In addition to avulsion injuries at the point of emergence from the dorsolateral midbrain, damage may be caused by compression of the nerve against the rigid tentorial edge as it passes anteriorly through the ambient cistern.
CNVI is tethered rostrally by Dorello’s canal, where most traumatic injuries occur.
The usefulness of CT and MRI is demonstrated in identifying blood within the ambient cistern in relation to avulsion injuries of CNIV. MRI also demonstrates hemorrhage at the point of emergence of CNIII in similar injuries. Fluid-attenuated inversion recovery (FLAIR) and gradient-echo sequences may be particularly sensitive in these scenarios.
Secondary injuries
In addition to primary injuries, the ocular motor nerves are susceptible to secondary injury as a result of raised intracranial pressure (ICP) leading to transcompartmental herniation. This is true of any etiology of raised ICP, including supra- and infratentorial space-occupying lesions, idiopathic intracranial hypertension, and venosinus thrombosis. CNVI reportedly is the nerve affected most commonly. In inferior transtentorial herniation, the proposed pathophysiology is descent of the brainstem resulting in necrosis of the nerve at Dorello’s canal or compression of the nerve against the clivus or BA. CNVI also is vulnerable to shifts in intracranial contents resulting from low ICP for similar reasons. In CNIII palsy, the mechanism is believed the result of impingement on the free edge of the tentorium or kinking over the clivus. The peripherally distributed parasympathetic pupilloconstrictor fibers are most vulnerable and mydriasis may be the earliest manifestation.
Cavernous
Injuries to the cavernous portion of the ocular motor nerves may result directly from fractures of the skull base. As CNVI traverses the cavernous sinus, it is tethered to the lateral wall of the ICA and vulnerable to indirect traumatic injury.
Orbital Apex
Traumatic orbital apex syndrome usually arises in the context of penetrating trauma and fractures but may occur as a delayed phenomenon resulting from the retention of foreign bodies. Iatrogenic injury also is described in the context of sinonasal and periorbital surgery.
Neoplasia
Nuclear/Fascicular
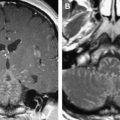
Any neoplasm involving the nuclear or fascicular component of the nerves within the midbrain and brainstem may give rise to ocular motor nerve dysfunction. Tumors recognized as causing nuclear palsies include metastases, brainstem and midbrain gliomas, medulloblastomas, and cavernous hemangiomas. Although such lesions may produce multiple neurologic deficits, some series report isolated ocular motor nerve palsies more commonly and have described them as secondary to metastases, gliomas, and cavernous hemangiomas ( Fig. 8 ).