Imaging of the trigeminal nerve requires a thorough understanding of its anatomy and function, clinical symptoms related to malfunction, and its key pathologies. Because of the nerve’s long course from the brainstem nuclei to the peripheral branches, MR imaging and CT studies have to cover a large anatomic area while providing high-resolution images. Although MR imaging has almost completely replaced CT as the diagnostic modality of choice for investigating trigeminal neuropathy, CT still plays a role in the assessment of skull base foramina and facial skeleton. In this article, the clinical, radiologic, and pathologic features of the most common conditions causing trigeminal nerve dysfunction at each specific anatomic level are discussed.
Various benign and malignant conditions may affect the trigeminal nerve, resulting in trigeminal nerve dysfunction (trigeminal neuropathy). Trigeminal neuropathy may be the only, the first, or one of several clinical signs of an underlying disease, leading the patient to seek medical advice. Based on clinical examination alone, it is not always possible to locate a lesion along the course of the trigeminal nerve precisely, because the trigeminal nerve has complex branching and anastomotic patterns. In addition, the underlying cause of trigeminal neuropathy cannot always be found based on clinical and laboratory findings alone. Therefore, trigeminal nerve dysfunction has become a common indication for imaging. The purpose of this article is to discuss the most common symptoms related to trigeminal pathology and their implications for imaging; to review the lesions involving the trigeminal nerve at specific levels, with special emphasis on clinical–radiologic correlation; and to demonstrate a spectrum of pathologic conditions, as observed on MR and CT images. Knowledge of the imaging features of these pathologic conditions can prevent their misinterpretation and facilitates therapeutic choice.
Imaging techniques
Although CT and MR imaging have a complementary role in the evaluation of trigeminal neuropathy, allowing evaluation of bony detail and soft tissues, respectively, MR imaging is, in many instances, the only imaging modality needed to provide the diagnosis. MR imaging is uniquely suited to image the entire course of the trigeminal nerve, from the brainstem nuclei, through the basal cisterns, within the skull base foramina, and in the suprahyoid neck. As suggested by several investigators, the diagnostic yield of imaging mostly depends on the quality of the examination and on the appropriate tailoring.
To image trigeminal neuropathy, high-field units (1 T–3 T) are mandatory because they provide a better signal-to-noise ratio, a better spatial resolution, and a shorter examination time. When performing an MR imaging examination of the trigeminal nerve, the challenge consists of performing high-resolution studies with smaller fields of view on one hand and covering the entire presumptive anatomic area of interest on the other hand, while keeping the duration of the MR imaging study within a reasonable time frame. High-resolution MR images can be obtained using parallel imaging techniques, surface coils, thin slices (0.5–2.0 mm), and the highest matrix size (384–512) for a minimal field of view, resulting in an in-plane spatial resolution of 0.4 mm to 0.7 mm. The nuclear and supranuclear portion of the trigeminal nerve are imaged using a standard brain protocol, including T2-weighted (T2-w), fluid-attenuated inversion recovery (FLAIR), diffusion-weighted, and T1-weighted (T1-w) images before and after intravenous administration of contrast material. The cisternal portion of the trigeminal nerve is best imaged using three-dimensional (3D) heavily T2-w sequences, such as 3D gradient-echo steady-state free precessing (SSFP) sequences, constructive interface in steady state (CISS) sequences, fast imaging employing steady-state acquisition [FIESTA], true fast imaging with steady-state precession [TrueFISP], balanced fast field echo [bFFE]) or, more recently, fast spin echo 3D sequences with optimized contrast using flip angle evolution (driven equilibrium [DRIVE], sampling perfection with application optimized contrasts [SPACE]). These techniques provide an excellent cerebral spinal fluid–nerve contrast and an excellent resolution because of thin slices (0.35–0.7 mm). Multiplanar reconstructions and 3D and virtual endoscopic views generated from these 3D data sets are extremely useful in assessing neurovascular conflicts in conjunction with 3D time of flight (TOF) MR angiography and contrast-enhanced MR angiography. Recently, diffusion tensor imaging has been used to assess the tissue integrity of the trigeminal nerve, with diffusion tensor imaging–derived fractional anisotropy (FA) being calculated for the cisternal portion of the trigeminal nerve. Meckel’s cave, cavernous sinus, skull base, and extracranial portions of the trigeminal nerve are imaged using high-resolution 2 to 3-mm thin spin echo (SE) or fast spin echo T1-w images in the axial and coronal plane pre- and postcontrast, and 3D T1-w sequences with 1-mm thin slices, such as 3D spoiled gradient echo, Magnetized Prepared Rapid Gradient Echo (Ultrafast Gradient Echo 3D sequence with magnetization transfer), or 3D Volume Interpolation Breath hold Enhancement, which allow multiplanar reconstructions in any chosen plane. Fat saturation techniques after intravenous administration of contrast material are extremely useful in that they increase lesion conspicuity, allowing identification of subtle enhancement patterns along the course of the trigeminal nerve, especially in the cavernous and extracranial portions.
Volumetric acquisition achieved with 16, 32, or 64 multidetector CT scanners enhanced by various 3D volume rendering, virtual endoscopy, and two-dimensional (2D) multiplanar or curved reconstructions is capable of imaging most pathologic conditions of the trigeminal nerve. However, CT of the trigeminal nerve is not as sensitive as MR imaging in the brainstem, cisternal segments, and cavernous sinus. Nevertheless, CT is nearly equal to MR imaging at the level of the skull base foramina and in the suprahyoid neck. In addition to soft tissue algorithm reconstruction, bone algorithm reconstruction is required to assess better any widening or compromise of skull base foramina and facial bone involvement. Thin sections (0.3–1.5 mm), high-resolution images, and 2D reconstructions in the axial and coronal plane are mandatory. As with MR imaging, if CT is used alone, imaging of the entire course of the trigeminal nerve is essential, because clinical examination cannot always precisely locate a lesion along the course of the nerve.
At the authors’ institution, patients who have trigeminal neuropathy are imaged using a combination of MR imaging and CT. First, an MR imaging examination of the brain and face is performed and if pathology involves the skull base or bony structures of the face, a thin-slice CT with bone window algorithm and without administration of contrast material is added. Interpretation of findings will then be made from the combined information from both examinations.
The role of ultrasound and angiography in imaging trigeminal neuropathy is minor, because the former is used only to guide fine-needle aspiration biopsies, and the latter may be performed either to assess precisely arteriovenous malformations (AVMs) and dural fistulae or before interventional procedures.
Clinical symptoms
Symptoms arising from pathologic conditions involving the trigeminal nerve (trigeminal neuropathy) depend on the segment and division of the nerve that is affected. Because V1 (ophthalmic), V2 (maxillary), and V3 (mandibular) divisions have sensory fibers, patients typically complain of pain, burning, or anesthesia and analgesia in the regions innervated by any of the three divisions. In addition, when V1 is affected, the corneal reflex may be absent (nasociliary nerve), with an increased risk of corneal injury and ulceration (neurokeratitis). When V3 is affected, patients may complain of weakness in chewing and deviation of the jaw on mouth opening (muscles of mastication), or they may present with serous otitis media (eustachian tube dysfunction from malfunction of the tensor veli palatini muscle).
Pathologic processes affecting the cavernous sinus may present clinically with ophthalmoplegia (cranial nerves III, IV, and VI), anisocoria or mydriasis (III), V1 and V2 deficits, chemosis, and proptosis.
Pathologic processes affecting the cisternal portion of the trigeminal nerve may result in trigeminal neuralgia (TN). TN, or tic douloureux, is the most frequently occurring condition of all nerve pain disorders. The pain, which comes and goes, feels like bursts of sharp, stabbing, electric shocks in the V1 or V2 distribution of the trigeminal nerve, and can last from a few seconds to a few minutes. Although the onset of TN is most often seen in middle or old age, young adults and children can also be affected. People with “classic” or “idiopathic” TN become plagued by intermittent severe sharp or stabbing pain that interferes with common daily activity. They live in fear of unpredictable painful attacks, which may lead to sleep deprivation, irritability, severe anticipatory anxiety and depression, dehydration, and malnutrition. A characteristic feature of TN is the trigger zone, a small area in the central part of the face, usually on a cheek, nose, or lip, that, when stimulated, triggers a typical burst of pain. A light touch or vibration is the most effective trigger. Because of this, common daily activities, such as brushing the teeth, shaving, eating, drinking, or talking, trigger the attacks. Typically, attacks are similar in individual patients and no neurologic deficits are clinically evident. Between paroxysmal attacks, most patients usually become relatively pain free. However, in time, the pain usually becomes more intense and the attacks more frequent. In some cases, the pain does not exactly fit these criteria; patients may experience a persistent ache between paroxysms (steady component) or mild sensory loss. Such disease has been labeled “atypical” TN. Less commonly, autonomic symptoms, such as conjunctival injection, lacrimation, eyelid edema, and nasal congestion as features of trigeminal autonomic cephalgias may be seen in association with TN. Although the clinical diagnosis of TN is straightforward in most cases, other causes of facial pain, such as dental pain, temporomandibular joint pain, migraine, temporal arteritis, trigeminal deafferentation pain, and atypical facial pain should be eliminated in patients in whom a paroxysmal and a steady pain component are noted. As opposed to TN, trigeminal deafferentation pain is pain that results from intentional injury to the trigeminal nerve system in an attempt to treat TN, particularly after destructive neurosurgical intervention. Numbness of the face and excruciating facial pain, sometimes accompanied by trophic wounds in the areas innervated by the trigeminal nerve, are a constant part of this syndrome, which has also been referred to as anesthesia dolorosa. Atypical facial pain, also called persistent idiopathic facial pain, is characterized by steady aching or throbbing pain, described as pressure and a sensation of swelling in the face. It has a strong female predominance, with typically no trigger zone on the face.
Diseases affecting the brainstem and the upper spinal cord nuclei may result not only in trigeminal neuropathy but also in other cranial nerve deficits (III, IV, VI, VII–XII) and brainstem syndromes, such as the lateral medullary syndrome (Wallenberg syndrome) and the medial medullary syndrome, and less common syndromes, such as Parinaud syndrome or Foville’s syndrome. A detailed discussion of these syndromes is, however, beyond the scope of this article.
Last but not least, some patients who have pathologic conditions of the trigeminal nerve may not have TN, facial pain, or numbness at all, and trigeminal pathology is discovered incidentally at imaging.
Clinical symptoms
Symptoms arising from pathologic conditions involving the trigeminal nerve (trigeminal neuropathy) depend on the segment and division of the nerve that is affected. Because V1 (ophthalmic), V2 (maxillary), and V3 (mandibular) divisions have sensory fibers, patients typically complain of pain, burning, or anesthesia and analgesia in the regions innervated by any of the three divisions. In addition, when V1 is affected, the corneal reflex may be absent (nasociliary nerve), with an increased risk of corneal injury and ulceration (neurokeratitis). When V3 is affected, patients may complain of weakness in chewing and deviation of the jaw on mouth opening (muscles of mastication), or they may present with serous otitis media (eustachian tube dysfunction from malfunction of the tensor veli palatini muscle).
Pathologic processes affecting the cavernous sinus may present clinically with ophthalmoplegia (cranial nerves III, IV, and VI), anisocoria or mydriasis (III), V1 and V2 deficits, chemosis, and proptosis.
Pathologic processes affecting the cisternal portion of the trigeminal nerve may result in trigeminal neuralgia (TN). TN, or tic douloureux, is the most frequently occurring condition of all nerve pain disorders. The pain, which comes and goes, feels like bursts of sharp, stabbing, electric shocks in the V1 or V2 distribution of the trigeminal nerve, and can last from a few seconds to a few minutes. Although the onset of TN is most often seen in middle or old age, young adults and children can also be affected. People with “classic” or “idiopathic” TN become plagued by intermittent severe sharp or stabbing pain that interferes with common daily activity. They live in fear of unpredictable painful attacks, which may lead to sleep deprivation, irritability, severe anticipatory anxiety and depression, dehydration, and malnutrition. A characteristic feature of TN is the trigger zone, a small area in the central part of the face, usually on a cheek, nose, or lip, that, when stimulated, triggers a typical burst of pain. A light touch or vibration is the most effective trigger. Because of this, common daily activities, such as brushing the teeth, shaving, eating, drinking, or talking, trigger the attacks. Typically, attacks are similar in individual patients and no neurologic deficits are clinically evident. Between paroxysmal attacks, most patients usually become relatively pain free. However, in time, the pain usually becomes more intense and the attacks more frequent. In some cases, the pain does not exactly fit these criteria; patients may experience a persistent ache between paroxysms (steady component) or mild sensory loss. Such disease has been labeled “atypical” TN. Less commonly, autonomic symptoms, such as conjunctival injection, lacrimation, eyelid edema, and nasal congestion as features of trigeminal autonomic cephalgias may be seen in association with TN. Although the clinical diagnosis of TN is straightforward in most cases, other causes of facial pain, such as dental pain, temporomandibular joint pain, migraine, temporal arteritis, trigeminal deafferentation pain, and atypical facial pain should be eliminated in patients in whom a paroxysmal and a steady pain component are noted. As opposed to TN, trigeminal deafferentation pain is pain that results from intentional injury to the trigeminal nerve system in an attempt to treat TN, particularly after destructive neurosurgical intervention. Numbness of the face and excruciating facial pain, sometimes accompanied by trophic wounds in the areas innervated by the trigeminal nerve, are a constant part of this syndrome, which has also been referred to as anesthesia dolorosa. Atypical facial pain, also called persistent idiopathic facial pain, is characterized by steady aching or throbbing pain, described as pressure and a sensation of swelling in the face. It has a strong female predominance, with typically no trigger zone on the face.
Diseases affecting the brainstem and the upper spinal cord nuclei may result not only in trigeminal neuropathy but also in other cranial nerve deficits (III, IV, VI, VII–XII) and brainstem syndromes, such as the lateral medullary syndrome (Wallenberg syndrome) and the medial medullary syndrome, and less common syndromes, such as Parinaud syndrome or Foville’s syndrome. A detailed discussion of these syndromes is, however, beyond the scope of this article.
Last but not least, some patients who have pathologic conditions of the trigeminal nerve may not have TN, facial pain, or numbness at all, and trigeminal pathology is discovered incidentally at imaging.
Pathologic conditions of the trigeminal nerve
For the purpose of this article, the clinical, radiologic, and pathologic features of the most common conditions affecting the trigeminal nerve are reviewed at each specific anatomic level: brainstem, cisternal, Meckel’s cave, cavernous sinus, and peripheral divisions. Various vascular, infectious and inflammatory, neoplastic, traumatic, and congenital conditions are discussed for each specific anatomic site.
Brainstem
Vascular lesions
The brainstem is the site of approximately 25% of acute strokes. Infarction at this site may cause diverse and complex patterns of dysfunction, depending on the site of the lesion and its relationship to the cranial nerve nuclei, long tracts, and brainstem connections. Most brainstem strokes are seen in patients over 60 years of age. At MR imaging, most patients have solitary acute ischemic brainstem lesions. However, as many as 15% have additional cerebellar lesions and 10% have acute supratentorial ischemic stroke lesions. The most common brainstem infarcts are seen in the dorsolateral medulla (occlusion of the ipsilateral posterior inferior cerebellar artery (PICA)) and in the paramedian pons (occlusion of pontine branches of the basilar artery). Spinal cord infarction, which may present with trigeminal neuropathy by affecting the dorsally located trigeminal tracts, is less common than brainstem infarction. Since the introduction of diffusion-weighted imaging, which is particularly sensitive to ischemic changes, diagnosis of ischemic strokes in the brainstem and upper spinal cord has become standard in most institutions ( Fig. 1 ).
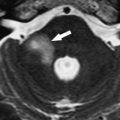
Hemorrhage involving the trigeminal nuclei and other cranial nerve nuclei may be caused by hypertension, cavernous angiomas, AVMs, or trauma (Duret’s hemorrhage). As a cause of hemorrhage, cavernous angiomas are far less common than hypertension; nevertheless, they must be excluded as a cause of hemorrhage, especially in young patients. Cavernous angiomas are considered to be congenital vascular hamartomas composed of endothelial-lined sinusoidal collections without significant amounts of interspersed neural tissue. A sporadic form and a familial form of disease with a genetic component have been observed. Cavernous angiomas are frequently located in the brainstem and have a characteristic MR imaging appearance caused by the presence of blood degradation products ( Fig. 2 ).
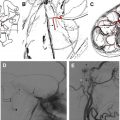
Brainstem AVMs are less common than cavernous angiomas and can be divided into pial lesions and parenchymal lesions. Pial AVMs typically receive arterial input from dilated branches of the superior cerebellar artery or the anterior inferior cerebellar artery and drain into the prepontine or petrosal sinuses. Because they are restricted to the pial surface, they can sometimes be completely resected. In contrast, parenchymal brainstem AVMs receive arterial inflow from brainstem perforating vessels, show a deep insinuation pattern within vital neural tissue, and drain through venous channels of the brainstem substance, which then empty into the petrosal sinus and galenic system. Therefore, safe microsurgical extirpation is not possible in most cases. The characteristic radiologic aspect of a brainstem AVM is seen in Fig. 3 .
Duret’s hemorrhages are delayed, secondary brainstem hemorrhages. They occur in craniocerebral trauma victims with rapidly evolving descending transtentorial herniation. In most cases, the outcome is fatal. On the basis of recent observations, it is believed that arterial hypertension and advanced age are risk factors for the development of Duret’s hemorrhage. The diagnosis is made on CT or MR imaging of the brain.
Inflammatory and infectious lesions
Demyelinating diseases of the white matter may affect one or more cranial nerve nuclei located in the brainstem. Multiple sclerosis (MS) is the single most common pathologic entity leading to trigeminal neuropathy, which may be the first presenting symptom or may be found in association with other cranial nerve deficits. The diagnosis of MS is based on clinical presentation (optic neuritis, transverse myelitis, internuclear ophthalmoplegia, paresthesias, and other neurologic abnormalities), cerebral spinal fluid analysis, and typical findings on MR imaging. MS plaques are hyperintense on T2-w and FLAIR images and hypointense on T1-w sequences. Specific signal intensities of MS lesions vary, depending on the magnetic field strength, the pulse sequence parameters, and partial volume effects. Occasionally, acute plaques may have a thin rim of relative T2 hypointensity or T1 hyperintensity. The T1 hyperintensity is attributed to free radicals, lipid-laden macrophages, and protein accumulations. Most MS plaques are small and irregular, but larger lesions can coalesce to form a confluent, tumefactive pattern. Active plaques may enhance after intravenous administration of contrast material because of transient breakdown of the blood–brain barrier 8 to 12 weeks after acute demyelination, whereas old plaques typically do not enhance ( Fig. 4 ). Isolated tumefactive MS plaques may mimic mass lesions and should be differentiated from other inflammatory or neoplastic lesions.
Various other inflammatory and infectious diseases may produce lesions similar to those of MS in the brainstem, such as acute disseminated encephalomyelitis, progressive multifocal leukoencephalopathy, HIV encephalitis, Behcet’s disease, Lyme disease, progressive neurosarcoidosis, Whipple’s disease, Bickerstaff’s brainstem encephalitis, vasculitis due to systemic lupus erythematosus, and listeria rhombencephalitis. Listeria rhombencephalitis–causing TN may occur in otherwise healthy subjects, and should be looked for in a specific endemic context ( Fig. 5 ). On MR imaging, transient enhancement of brainstem nuclei is observed.
Neoplasms
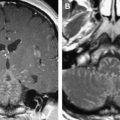
The trigeminal nuclei may be affected by gliomas, lymphomas, and hematogenous metastases to the brainstem. Brainstem gliomas account for 10% to 20% of all childhood brain tumors. The incidence in adults is lower than that in children younger than 16. In adults, brainstem gliomas follow a more indolent course than in children and are more likely to remain localized. Diffuse glioma is the most common form of brainstem glioma. It leads to progressive cranial nerve deficits, often asymmetric, and trigeminal symptoms are only rarely striking clinical features. Long tract signs and ataxia of trunk and limbs are common, whereas hydrocephalus is uncommon, even with large tumors. The histology of brainstem glioma is variable, although most of these tumors are of low-grade malignancy. Almost all occur in the pons but they may spread in any direction into the mesencephalon or medulla ( Fig. 6 ) or may show exophytic growth that may encase and elevate the basilar artery. The typical MR imaging appearance of a brainstem glioma is an expansive, infiltrative process with low-to-normal signal intensity on T1-w images and heterogeneous high-signal intensity on T2-w and FLAIR images, with or without contrast enhancement.
The most frequent metastatic tumors to the brain are bronchogenic carcinoma and breast carcinoma. Most commonly, hematogenous metastases are seen as multiple lesions located at the gray-white corticomedullary junction. Less frequently, metastases may be found in the brainstem, leading to cranial nerve deficits. Enhancement is characteristic for most lesions.
Congenital lesions
Congenital lesions involving the trigeminal nerve are rare and include aplasia and hypoplasia of the nerve and brainstem nuclei. Aplasia and hypoplasia of the trigeminal nerve have been described in association with Goldenhar-Gorlin syndrome, also called oculo-auriculo-vertebral syndrome, or as an isolated lesion.
Cisternal (Preganglionic) Segment
Vascular lesions
Increasing evidence suggests that the most frequent cause (80%–90%) of classic TN is compression of the trigeminal nerve root close to its point of entry into the pons (root entry zone) by aberrant loops of arteries or veins, large or small, that may simply contact or indent the trigeminal nerve. The axons of the root entry zone of the trigeminal nerve are coated with central nervous system myelin, which extends up to 5 mm along the root into the prepontine cistern. In consequence, the junction between central nervous system myelin and peripheral nervous system myelin, which is approximately 2 mm in length, is located some distance away from the pons. Examination of trigeminal nerve roots from patients with compression of the nerve root by an overlying blood vessel has revealed focal demyelination in the region of compression, which results in the abnormal generation of ectopic sensory impulses with subsequent spread to adjacent fibers, so that spontaneous nerve activity increases. Over time, this nerve activity is thought to cause hyperactivity of the trigeminal brainstem nucleus, resulting in the generation of TN pain. Decompression of the nerve root by surgery produces rapid and long-term relief of symptoms in most patients who have vessel-associated TN, and intraoperative assessments report immediate improvement in trigeminal conduction on decompression. This phenomenon is thought to reflect the reversal of compression-induced conduction block in larger myelinated fibers outside the region of demyelination. Similarly, vascular compression of the facial and glossopharyngeal nerves is thought to be responsible for most cases of hemifacial spasm and glossopharyngeal neuralgia, respectively.
In classic TN, the superior cerebellar artery or one of its branches, the anterior inferior cerebellar artery, the PICA, or the basilar artery, usually compresses the root entry zone. Rarely, the conflicting vessel is a petrous vein, a saccular aneurysm, a persistent trigeminal artery, or an AVM. Recently, it has been shown that the size of the cerebellopontine cistern, as measured with MR imaging, may play a role in favoring a neurovascular conflict, because a significantly smaller volume of the cistern was found in patients who had TN on the affected side.
Because contact between the cisternal portion of the trigeminal nerve and a blood vessel may also be seen in asymptomatic patients, the diagnosis of a neurovascular conflict should only be made at MR imaging with caution. Only in the presence of an appropriate clinical setting, if the site of contact is the root entry zone, the offending vessel crosses the nerve perpendicularly, the nerve itself is deviated or indented by the respective vessel, or it is encased by two or more vessels should the diagnosis of neurovascular compression be made on MR imaging ( Fig. 7 ). Using high-resolution MR angiography and 3D gradient echo SSFP sequences, the areas of compression and the conflicting vessels can be identified preoperatively. Recently, it has been shown that neurovascular conflicts can be identified at MR imaging in 88% of the typical TN cases and in 56% of the atypical TN cases. Although arterial compression is much more common than venous compression (90% of cases with typical TN and 52% of cases with atypical TN), pure venous compression is seen more often in patients who have atypical TN (24%) than in patients who have typical TN (1%). At many institutions, MR imaging is involved nowadays in the decision making when considering microvascular decompression because it can differentiate between the types of compression; in MR imaging–negative cases for atypical TN, treatment modalities other than microvascular decompression can be considered.